|
COVID-19 has been transmitted to 192 countries and regions. Unlike the severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), which have relatively high fatality rate, COVID-19 has a high infection rate but is less lethal. The earliest variant, named Alpha (α), was identified in the UK in September 2020, while Beta (β) and Gamma (γ) were identified in South Africa and Brazil in September 2020 and October 2020, respectively. The Eta (η) and Delta (δ) lineages were both identified in November 2020 in the UK and India, respectively. The Omicron (o) lineage was discovered in November 2021 in South Africa. Compared to the original lineage, the new coronavirus variants show a higher transmissibility and resistance to antibodies.
Coronavirus Disease 2019 (COVID-19) has caused tremendous losses to the world. Influenza and the four human coronaviruses (OC43, 229E, NL63, and HKU1) that cause common colds are endemic. But we need annual vaccines and acquired immunity without requiring lockdowns, masks and social distancing. As the virus persists, people develop some immunity to it and they don’t come down with severe symptoms.
A large study of people who have had COVID suggests that their levels of neutralizing antibodies start to decline after around six to eight months. But their bodies also make memory B cells, which can manufacture antibodies if a new infection arises, and T cells that can eliminate virus-infected cells. A vaccine that is 90% effective at blocking transmission will need to reach at least 55% of the population to achieve temporary herd immunity, in addition to some social distancing measures, plus the use of face masks and having as many people as possible working from home.
But if the rate of transmission increases because of a new variant, or if a vaccine is less effective than 90% at blocking transmission, then vaccine coverage will need to be greater to blunt circulation. COVID will be eliminated from some countries, but with a continuing risk of reintroduction from places where vaccine coverage and public health measures have not been good enough.[1]
There are at least eight regions that have reported the discovery of the new coronavirus variants, so far, namely California (USA), Kent (UK), Manaus (Brazil), New York (USA), Kampala (Uganda), Maharashtra (India), Nelson Mandela Bay (South Africa), and Osun (Nigeria).
The new variants are located in almost every continent. Previous studies show that the optimal temperature zone for spreading the coronavirus is between 5 and 15 ∘C. The cold weather and mass gatherings have accelerated the spread of the virus, and the new coronavirus variants also increase the transmission rate. A significant correlation between neutralizing antibody titers and anti-receptor binding domain IgG antibody titers.
Identifying immune correlations of protection from COVID-19 is critical to predicting how the expected antibody decay will affect clinical outcomes, if and when a booster dose will be needed, and whether vaccinated persons are protected. Such capacity for prediction is particularly important for new vaccine development. [2]
COVID- 19 infection in humans may lead to symptomatic or asymptomatic cases. Infection shows diverse symptoms ranging from mild to severe.
A neutralizing antibody cocktail (casirivimab-imdevimab) is a formulation of 2 recombinant human immunoglobulin G1 monoclonal antibodies that targets the receptor-binding domain of the spike protein of severe acute respiratory syndrome coronavirus-2, blocking the viral attachment and entry into human cells.
Patients with mild-to-moderate COVID-19 who are at a high risk for progression to severe disease received this treatment. None experienced progression of symptoms or required hospitalization due to COVID-19.[3,4,5 ]
Ivermectin has been proposed to mediate its antiviral effects via host cell mechanisms, namely inhibition of the importing of α/βmediated nuclear transfer of viral cargo proteins. Another mechanism of action has been proposed, assuming its role as an ionophore agent. Ionophores have many interal oxygen atoms and are essential for binding cations and transporting them through phospholipid bilayers (cell membranes; phospholipid capsid of the virus). As a consequence, it determines an ionic imbalance between the external and internal environment, with the consequent osmotic lysis. Ivermectin may be an excellent adjuvant method for Personal Protective Equipment, for the prophylaxis of COVID-19 in health personnel and their contacts. Antiviral activity was assessed following continuous exposure to serial dilutions of ivermectin, which caused concentration dependent antiviral effects with practically total eradication at 5μmol/L and half maximal inhibition at approximately 2.5μmol/L. We need the clinically applied dosage schedules to get good outcome. [6,7,8 ]
Remdesivir (GS-5734), an inhibitor of the viral RNA-dependent, RNA polymerase with in vitro inhibitory activity against SARS-CoV-1 and the Middle East respiratory syndrome (MERS-CoV), was identified early as a promising therapeutic candidate for Covid-19 because of its ability to inhibit SARS-CoV-2 in vitro. In addition, in non-human primate studies, remdesivir initiated 12 hours after inoculation with MERS-C0V reduced lung virus levels and damage. [9]
MK-4482, an orally available pro-drug of the nucleoside analogue N4-hydxycytidine (NHC), which has shown potent anti-influenza virus activity in mice, guinea pigs, ferrets, and human airway epithelium organoids. Acting through the induction of error catastrophe in virus replication, NHC has broad-spectrum anti-RNA virus activity. MK-4482 has been shown to function as an RNA mutation-inducting genome catastrophe. [10 ]
The combined treatment of MK-4482 and GS-5734 results in a marked potentiation of efficacy in a COVID-19 hamster infection model through an increased frequency of mutations in viral genome. [11 ]
A prophylactic vaccine acts by displaying unique antigens from virus to the immune system, seeking out to train the immune system for the expeditious generation of a significant number of antibodies at the time of actual infection.
For the immune system, events that induce memory include infections with viruses and bacteria as well as vaccinations. We will encounter many infectious threats -- some once and others multiple times. But if the first encounter created an immunologically protective state that persists, these repeat exposures can pass essentially unnoticed, producing no or very mild symptoms of infection.
Memory is cardinal feature of so-called adaptive immune responses, which are those involving lymphocytes, key cells of the immune system. When an infection triggers a lymphocyte response, it induces a small number of pre-existing B and T cells to proliferate, creating a small army of cells specific for -- and thus able to combat -- the infection agent. B and T cells are triggered to respond by recognizing a part of the pathogen through their antigen receptors with a certain strength.
Once the pathogen has been subdued and the response is effectively over, most pathogen-specific B and T cells die, but a small number of these recent combatants-B cells, T cells antibody-secreting plasma cells-persist as specialized, long-lived immune memory cells. [12 ]
Immunity is a multifaceted phenomenon. For T cell-mediated memory responses to COVID-19, it is relevant to consider their impact both on COVID-19 disease severity and on viral spread within a population. We may reflect on the immunological and epidemiological aspects and implications of pre-existing cross-reactive immune memory to COVID-19, which largely originates from previous exposure to circulating common cold coronaviruses.
For SARS-CoV-2, a member of the coronaviruses HCoV-OC43, HCoV-HKU1, HCoV-229E and HCoV-NL63, the extent and nature of cross-reactivity of immune responses are variables that can influence the near and long-term trajectory of the current pandemic. T cell immunity does not prevent infection but, rather, can modulate the time course of the disease and its infectiousness.[13]
Vaccines can be prophylactic or therapeutic and can broadly be classified as live attenuated vaccines, inactivated vaccines, subunit vaccines or toxoid vaccines. Subunit antigens often display lower immunogenicity, which can be rectified by employing delivery systems and/or compounds as adjuvants to boost immunogenicity.[14]
As protective immunity does not exist in humans and the virus is capable of escaping innate immune responses, it can proliferate, unhindered, in primarily infected tissues. Subsequent cell death results in the release of virus particles and intracellular components to the extracellular space, which result in immune cell recruitment, the generation of immune complexes and associated damage. Infection of monocytes/macrophages and/or recruitment of uninfected immune cells can result in massive inflammatory responses later in the disease. Uncontrolled production of pro-inflammatory mediators contributes to acute respiratory distress syndrome and cytokine storm syndrome. [15]
In COVID-19, inflammatory microvascular thrombi are present in the lung, kidney, and heart, containing neutrophil extracellular traps associated with platelets and fibrin. Patients with COVID-19 also present with neutrophil-platelet aggregates and distinct neutrophil activation patterns in blood, which changes with disease severity. The risk of cerebral venous sinus thrombosis with COVID-19 infection is far greater than that associated with vaccination. [16,17,18 ]
The S protein is found on the surface of the virus and receptor-binding domain helps in the attachment of the virus to host cells whereas the N protein is an abundant protein and involved in the transcription, replication, and packaging of the viral genome. Development in the technologies has been created to enhance the stability and the efficacy of protein expression in mRNA or other nucleic acid-based vaccination. However selection or design of the antigen is essential. The neutralizing antibody titer after vaccination is a marker of overall immune response and suggests a possible role for the IgG titer. [19]
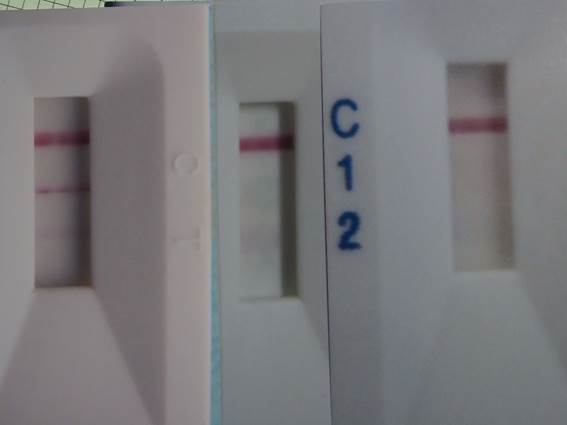
Fig.1: One month (left), three months (center) and six months (right) after treatment. Upper line: control, second line: IgG, lower line: IgM.
The nasal application with the solution of leuco-Methylene Blue (15μmol/L) in the presence of L-Ascorbic Acid ( 4.5m mol/L) may kill a member of coronaviruses.[20]
Rapid diagnostic testing can detect IgG antibodies after the treatment. This assays the blood and provides monthly results. The IgG antibody gradually decreased up to 6 months. (Fig 1). No outpatients have had COVID-19 infection after this treatment.
References:
[1] N.Phillips ( 2021 ) The coronavirus is here to stay -- here’s what that means. Nature590,382
[2] Y.Zho, J.Huang,L.Zhang, et al.(2021) The global transmission of new coronavirus variants. https://www.sciencedirect.com/science/article/pii/S0013935121015413 Google Scholar
[3] A.Baum, R.Copin,D.Ajithdoss,et al.(2020) REGN-COV2 antibody cocktail prevents and treats SARS-COV-2 infection in rhesus macaques hamsters. hpps://doi.org/10.1101/2020.0802.233320 Google Scholar
[4] D.M.Weinreich,S.Sivapalasingam,T.Nortun, et al.(2021) REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N.Engl.Med.384,238
[5] D.Abbay, L.Stephen,W.Kein, et al. (2021) Casirivimab-imdevimab for Treatment of COVID-19 in Solid Organ Transplant Recipients: An Early Experience. Transplantation.105,68
[6] G.Momekov,D.Momekova.(2020) Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regiments. https://doi.org/10.1080/13102818.2020 Google Scholar
[7] R.R.Hirsch, E.Carvallo, Hector.(2020) Ivermectin as Prophylaxis Against COVID-19 Retrospective Cases Evaluation. Microbial Infect Dis.4,1
[8] V.D.Schmith, J.Zhou, L.R.L.Lohmer.(2020) The approved dose of Ivermectin alone is not the ideal dose for the treatment of COVID-19.Clin.Phamacol. & Therapeu.108,762
[9] J.H.Beigel, K.M.Tomashek,L.E.Dodd, A.K.Mehta,et al. (2020) Remdesivir for the Treatment of Covid-19 Final Report. N. Engl. J. Med,383,1813
[10] K.Rosenke, F.Hansen, B.Schwarz, et al. ( 2021 ) Orally delivered MK-4482 inhibits SARS-COV-2 replication in the Syrian hamster model. Nature Com. 12,2295
[11] R.Abdelnabi, C.S.Foo, S.J.F.Kapten, et al. (2020) The combined treatment of Molnupiravir and Favipiravir results in a marked potentiation of efficacy in a SARS-CoV2 hamster infection model through an increased frequency of mutations in the viral genome. https://doi.org/10.1101/2020.12.10.419242 Google Scholar
[12] I.Quast, D. Tarlinton ( 2012 ) B cell memory: understanding COVID-19. Immunity. 54,205
[13] M.Lipstch, Y.H.Grad, A.Sette, et al. (2020) Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nature Reviews Immunology. 20,709
[14] A.Wadhwa, A.Aljabbari, A.Lokras, et al. (2020) Opportunities and Challenges in the Delivery of mRNA-Based Vaccines. Pharmaceutics.12,102
[15] S.Felsenstein, J.A.Herben, P.S.McNamara, (2020) COVID-19: Immunology and Treatment options. Clin. Immunol. doi:10.1016/j.clim.2020.108448 Google Scholar
[16] L.Nicola, A.Leuning, S.Brambs, et al. (2020) Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 142, 1176
[17] K.L.Furie, M.Cushman, M.S.V.Elikind, et al. (2021) Diagnosis and Management of Cerebral Venus Sinus Thrombosis With Vaccine-Induced Immune Thrombotic Thrombocytopenia. Stroke 52,2478
[18] H.Morooka (2005) Advancement in neurology: Innovation in stroke treatment. www.bwwsociety.org/journal/achive/neurology.htm
[19] H.Bergwerk, T.Conen, Y. Lustig, et al. (2021) Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N.Engl.J.Med. 385,1474
[20] H.Morooka ( 2021) Prophylactic treatment for COVID-19 with Methylene Blue in the presence of Ascorbic Acid. https:bwwsociety.org/jounal/current/2021/prophylactic-treatment-covid-19-with-methylene-blue-in-the-presence-ascorbic-acid.htm