Useful and Questionable Applications
of Several Vitamins and Anti-Vitamins
by Prof
Dr Dr Randolph Riemschneider
Central Institute of
Chemistry, Universidade Federal de Santa Maria (UFSM), Santa Maria, Rio Grande
do Sul, Brazil
Editor’s Note: This retrospective
explains how, in the course of his research projects, the author, LLB Fellow,
had to ‘deal with’ vitamin problems more than once, for example with anti-B6
vitamins as a trigger for cannibalism (Topic 1), with the application of the vitamin
C complex as stabiliser (Topic 2) and the non-existence of "vitamin B15"
used for medical purposes despite the risk (Topic 3).
In two lectures on "Vitamins
and Antivitamins" in
Vitamins or vitamin complexes,
respectively, are organic compounds
which must be introduced into an organism in small quantities and cannot be synthesised
by this organism. They actively contribute to the intermediary metabolism. In
each case, a definition what this organism is must be made[1].
The vitamins B6
(pyridoxine, adermin) and C are complexes; B6 comprises pyridoxol, pyridoxamine
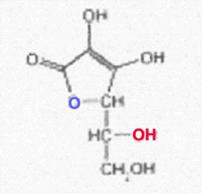
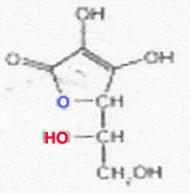
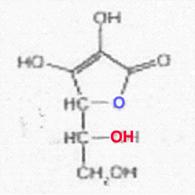
and pyridoxal (3b, 9b), the vitamin C complex consists of L-ascorbic acid [IIa]
plus dehydroascorbic acid [IIb] (3a).
In the above-mentioned lectures, all
of the vitamins and the anti-vitamins important for research were addressed.
Special attention was given to the determination of the overall vitamin C [IIa
plus IIb]. The methodology is described in (3a); cf. also Plate 2 in the
APPENDIX.
Topic 1
"Cannibalism in animals triggered suddenly by the oral
administration of the anti-B6 vitamin: 4-deoxy-pyridoxine (I)"
Definition of
cannibalism:
A cannibal is an animal that feeds
on other of its own species. This is not an unnatural characteristic: around
140 different species show cannibalistic tendencies under various conditions.
It occurs among mammals and birds, especially when food is scarce (4).
Cannibalism
triggered suddenly by the administration of I [4-deoxy-pyridoxine = 2-methyl-3-hydroxy-4-methyl-5-hydroxymethyl-pyridine
(9b)]
to pregnant mice of the strain NMRI and stopped immediately as soon as the
administration of I was discontinued
(5a,b).
The possibility to
create cannibalism and eliminate it again right away was detected by chance in
1955 when we obtained positive results in the study of "the influence of
the gender ratio of new-born rodents before and during copulation" (6) and
related this result to the vitamin B6 complex (B6-deficit), well aware that B6 is involved in reproduction.
Starting from the observation that more girls than
boys are born during war years as a result of the restricted diet [subsequently corroborated by
discussions with the Professor for Medical Statistics, Dr. Karl Freudenberg
(nicknamed "Kugelkarl)] we
carried out five series of experiments on mice over several years (1955 to
1962), first using 1000 ♂ to 4000♀ animals per experimental series
(6). In the experimental series, we "starved" the male mice by
keeping their body weight constant at 20 g over the entire period. Under these
circumstances, they were still strong enough to copulate. The condition was
that four female mice were available to one male mouse [a condition we had
established as early as 1938 in connection with other issues (7)]. In almost
all test series, a shift of the gender ratio to 70♀ to
30♂ was found when the males were "starved". Mouse
strain: NMRI.
One example for the many experiments
regarding cannibalism triggered by 4-deoxy-pyridoxine (I) carried out in
All in all, another 20 basic series
of experiments with I [each of which with 500 animals (400 female:
100 male)] were carried out over the years. The conditions [amount of I administered, time and duration of
the I application (other rodents
such as rats, golden hamsters, dormice)]
were varied (9e,d). Experimental runs followed to determine the possible
simultaneous influence of arginine and other amino acids, prolactin,
testosterone and other factors (9a,b) and others not mentioned.
As far as possible from a quantity
point of view, other anti-B6 vitamins and related compounds were
included in these experiments (9d). Unfortunately, the experiments relating to
the synthesis of I analogues turned
out to be time-consuming and complex. Experiments over several years on the improvement
and facilitation of the synthesis of I
also presented unforeseen difficulties[2]
(9c,d). The report on experiments carried with other animals such as rodents
and fish is included elsewhere.
Tabelle 1: Example of an experimental
report about the application of 4-deoxy-pyridoxine (I) to pregnant mice
(per 1 pregnant female in experiment respectively in controle)
Data for mouse number 127:
Application of I in water from day 14 to 21; delivery day :15; normal drinking water from day 1 to 13 and
from day 22
Experimental data:
Day : ……………………………………….13 14
15 16 17
18 19 20
21 22 23
24 25
Weight of animal :………………………… 51,5
50,1 29,6 29,6
27,2 27,8 27,9
27,6 27,3 32,7
35,5 34,8 34,8
Young number, reduced by cannibalism
: …………….13 11 9
7 7 6
3 3 3
3 3
Data for corresponding controle
mouse to 127: No application of I;
delivery on day 16; normal
drinking water all time
Experimental data:
Day……… ………….……………………….14 15
16 17 18
19 20 21
22 23 24
25
Weight of animal :
…………………………..50,3 50,4 31,2
32,1 33,7 33,8
33,7 34,1 34,0
34,2 34,5 34,4
Young Number
:…………………………………………….12 12 no reduction
Prescription of oral application
of I in drinking water to pregnat mice:
The experiment comprised 40 pregnant
mice each, 30 for the main test with I,
10 for controls. From day of delivery on every mother had its own cage and drinking
dish with pellet feed. The weight was checked daily.
I was offered in an aqueous solution in a
concentration between 5 and 25 g of I per
1000 ml. The animals consumed approx. 5 ml of liquid per day. The solution I was first administered to the
pregnant mice one day before birth and then for another 5 or 7 days. Feed and
drink ad libitum (9e,d).
The method described here, namely to create cannibalism "on
command" by administration of 4-deoxy-pyridoxine (I) is primarily useful
for research.
The question of whether it might be
used for the extermination of rodents
was pursued by us in several directions, but without satisfactory result so
far. We carried out:
1 Experiments
to improve the synthesis of I and to
make it cheaper (9c), as mentioned above, unfortunately without success.
However, this is not true for another analogue of I we synthesised; cf. 4.
2 Field
experiments with bait containing I so far had a success rate of only 40% to 50%: rodents also look for other feed.
3 Field experiments with
leaving bait containing no B6 at all had a success rate of only 20%
to 30% as long as the rodents were free to choose their feed.
4 Field
experiments with bait containing the analogue mentioned under point 1 above
showed a success rate of 70% to 80% so far. A 100% success is prevented by the
rodents being able to choose their feed.
It was possible to carry out these
field experiments in suitable institutions in
The author woud like to thank
Dipl.-Chem. H.-J.Hein, (diploma in chemistry), for his cooperation from 1955
until 1962 (5,6) in organizing and starting up the mice experiments and later
watching them over carefully.
Acknowledgment of the contribution of
Ms. Manjula Taneja , BSc., MSc. (
Comments on the unbelievable termination of the long-term cooperation by a
so-called student team3, cf. SPECIAL SECTION, Regarding: Topic 1
After Mrs.Taneja “banishment” by the
mentioned student team[3]
the author succeeded in continuing the work on the topic
"Cannibalism" - unfortunately only in part - at the Brazilian Federal
University Santa Maria, in Santa Maria, RS (UFSM) and with the aid of the local
industry. In addition to his work in Berlin (FU), the author established a
Central Chemical Institute at UFSM from 1963 to 1972 and was able to continue
some of his research projects both there as "Diretor-Coordenador" and in
In
Topic 2
"The vitamin C complex as a stabiliser"
This was triggered by the necessity
to stabilise pre-fabricated medical bulk merchandise such as SEREX[4]
before shipment to
The vitamin C complex formulated in
Plate 1, ascorbic acid [IIa] / dehydroascorbic acid [IIb] is stable if one
of the three factors H2O, O2 and metal ions can be
excluded. In the presence of O2 the component IIb is dominant in
aqueous vitamin C solutions, evident from the yellow colouring[5].
By adding vitamin C we have not only
stabilised SEREX4 before shipment in 1 l glass bottles, but also
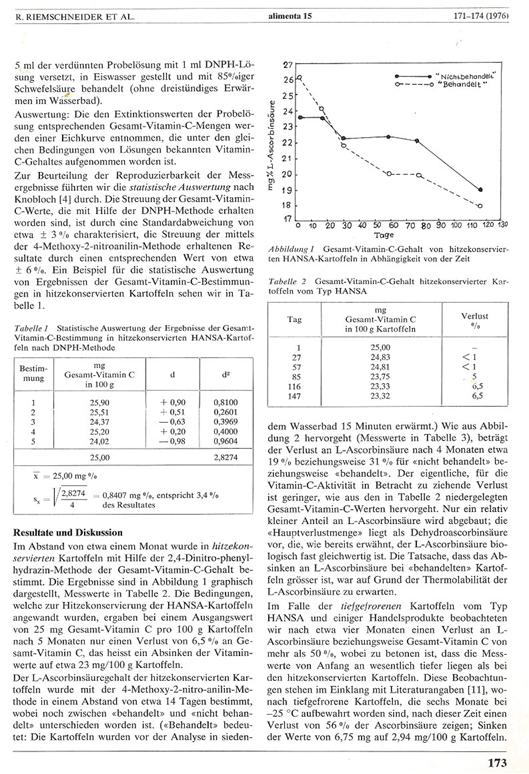
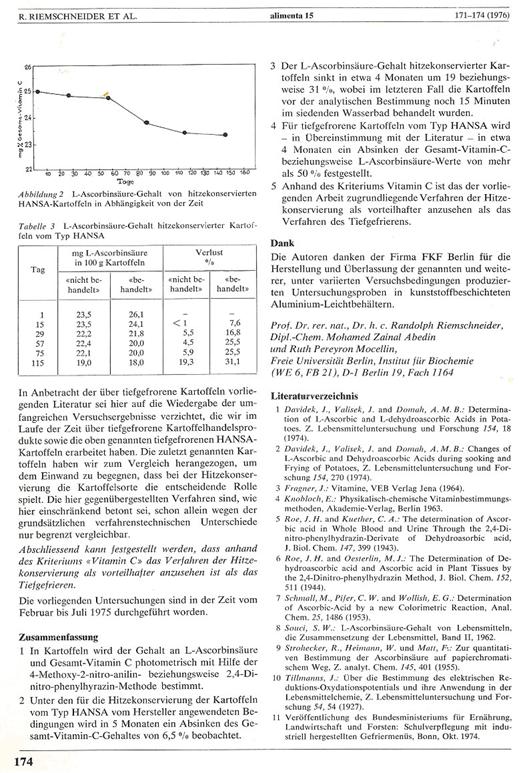
kept the vitamin C content of heat-preserved
potatoes stable by complete removal of O2 and adjusting a
desired vitamin C content:
The publication
dealing with this subject is shown as Plate 2 in the APPENDIX. However, it was not described in the paragraph
"preservation of potatoes by heat" on page 172 how stabilisation of the heat-preserved potatoes was really achieved.
At the express request of the manufacturer, the following sentence was left out
in the published text after the passage "containing 1.5 % NaCl as the main
component": "In order to
remove oxygen from the filled vessels to be sealed, the amount of vitamin C
calculated for oxygen (plus 10 %) was added." This was the only way to
ensure that the vitamin C content of the heat-preserved potatoes remained
stable over a longer period of time. (As mentioned earlier, vitamin C is
degraded only if all three of the factors necessary for the degradation of
vitamin O2, H2O and catalysing traces of metal are present.) For further details on this
topic, please refer to the cited publications and lectures. Quotations (10a –
h, and 11).
The competitors, i.e. the manufacturers
of deep-frozen potatoes became very agitated at the time and reacted
negatively, as the publications (10f,g) show. There were many telephone calls
from upset "customers". The reactions of the press were also very
varied. A lot of fuss could have been avoided at the time if the author had
been allowed to put all of his cards on the table (see above).
In closing, let me point out that,
in our case, there was no "vitaminisation" of potatoes.
Plate 1
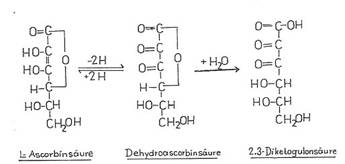
IIa IIb IIc
The vitamin C complex
consists of L-ascorbic acid [IIa] and
its primary product of oxidation, dehydroascorbic
acid [IIb], IIb is biologically practically equivalent to IIa, contrary to
2,3-diketo-gulonic acid [IIc] produced by hydrating IIb and opening the lactone
ring.
In the formulation
according to Emil Fischer, the C-atom which has the highest degree of oxidation
is given the number 1. According to the definition, the L-configuration is
evident from the C-atoms 5 and 6. Additional
information of monosaccharides and the terms
“D” and “L” in the essay “Stereochemistry of
Heptites” , there in Plate 4 “Kohlenhydratstammbaum” (D-Reihe) and in Plate 5
“Explanation of terms D and L”, published in this Journal (20); see also the monograph of the
author (8b).In Plate 3 (our APPENDIX) ,on the other hand, the formulation is
simply according to generation and derivatisation.
How did we come to dealing with
"heat-preserved potatoes" of the FKF company,
Patent attorney and certified
chemist Manfred Miehe who had written his Diplom thesis under the guidance of
the author at the FU submitted a problem that his client, FKF Berlin, was faced
with to his supervisor: How stable is the vitamin C content in potato preserves
developed by FKF? How can vitamin C be stabilised in "heat-preserved
food"?
Thanks to his experience with Serex4, the author saw a
solution which, as the publications cited under (10a-k, 11) show, was put into
practice within a few months. The above-mentioned medicament Serex4 aka Cellryl is a preparation developed by
the author and registered for clinical use, namely for the treatment of ulcers,
in Japan which was stabilised by the addition of an amount of L-ascorbic acid
calculated for O2 before export to Japan (8a).
Topic 3
"About the non-existence of the so-called vitamin B15 and B15-risk“
This was triggered by looking for
substances which, according to literature, have properties stimulating the
metabolism. Over 30 years, a systematic study of defined chemical
compounds as well as natural substances and preparations prepared from such
substances was carried out with regard to increase or decrease of
respiration[6]
in the WARBURG test or with regard to increasing the growth of tadpoles of Xenopus
larvis DAUDIN.
In the course of this search at the
time, we came across vitamin B15, the so-called pangaminic acid, to
which the Soviet authors had attributed an increase in metabolic activity.
However, this result did not
stand up when we repeated the test. Publication: "No cell respiration-promoting effect of vitamin B15
preparations. On the question of the non-existence of B15 aka
pangaminic acid." in Kosmetik-International 1983, H.4, p. 11 - 12,
based on the lab reports from (12a-c):
Both pangaminic acid preparations
bought commercially and those we made ourselves did not show the slightest trace of stimulating respiration of
liver homogenate. This was found both in our own WARBURG tests and in any other
common lab test procedures for detecting an increase in metabolic activity
during our research in the years 1977-82 (12).
Our own experiments and the subsequent in-depth study of literature
about B15 according to
Table 2: 271 literature passages revealed that everything connected to the
discovery and development of vitamin B15 can only be classified as
criminal. Neither the constitution nor
the vitamin character of B15 has ever been proven; more in Plate
4 (13-15).
Table 210:
Publications
about "pangaminic acid", "vitamin B15" and
derivatives thereof
Number
Topics
References in literature*)
23 Isolation, purification, synthesis,
patents 38 – 60
24 Analysis and identification 232 – 255
147 Tests on animals 61 – 207
18 Medical applications on humans
208 – 225
6 Applications in the field of
cosmetics, etc. 226 – 231
17 Summaries 256
– 271, 243
*) The references to
literature in Table 2 refer to the literature citation (13).
Unfortunately, more than 200 authors
did not notice this and, in most cases, arrived at the wrong conclusions. The
citations of all 271 publications we procured and translated where necessary
are found in the essay: "About the
undiscovered vitamin B15 (pangaminic acid)" (13), as are
the formulae given by the "discoverers" in the U.S. Patent 2,710,876
dated June 14, 1955. None of the
reproductions of this patent carried out in our labs by Thomas Wons, Klaus
Hennig, Gerhard Quelle and Heidi Horak over five years resulted in the products described (12a,b).
The medical application of
preparations containing "vitamin B15" or pangaminic
acid", respectively, as recommended in literature entails risks, as can be inferred from the
excerpt of the publication "Existence of pangaminic acid - risk" aka
"vitamin B15"?
shown in Plate 4 (13 - 15) in APPENDIX.
In der Literatur ist keine
durch Pangaminsäure und Derivate (alias „Vitamin B15“) zu beseitigende „B15-Avitaminose“
beschrieben, geschweige denn experimentell
bearbeitet worden, wie es für den Nachweis eines echten Vitamins
notwendig gewesen wäre.
Die 1913 von FUNK als Vitamine definierten organischen Verbindungen sind durch das Auftreten von speziellen Krankheiten (später als „Avitaminosen“ bezeichnet) entdeckt worden. Deren Heilung konnte geschehen, nachdem man die fehlenden lebensnotwendigen Stoffe in mühsamen, langwierigen Versuchen isoliert und identifiziert hatte: Vitamin B1 , identifiziert als Aneurin, heilend: Beri-Beri; - Vitamin C, identifiziert als Ascorbinsäure, heilend Skorbut ecc. Nichts dergleichen gibt es für „B15“ .
Also „Hände weg“ von angepriesenen Pangaminsäure-Vitamin-Präparaten !
In the following SPECIAL
SECTION, additional issues connected
with the topics 1 and 2 will be discussed and also the Plates 2-8 in the APPENDIX.
SPECIAL SECTION
Regarding Topic 1:
How the application of anti-B6 vitamins ever came about, i.e.
how the properties of 4-deoxy-pyridoxine (I)
which trigger cannibalism were discovered in 1955.
The influence on the gender ratio of
new-born rodents before and during copulation was studied in five experimental
series from 1955 to 1962 on 5000 mice each (♀ : ♂ = 4 : 1). In the
experimental series where we "starved" the male mice, i.e. kept their
body weight constant at 20 g, the gender ratio of the new-born mice was 70 :
30. These results were confirmed in further test series.
In some tests we reduced the B6
content in the diet of the mice and gave anti-B6 vitamin I (5a). In the test series with other
anti-B6 vitamins synthesized in the meantime we were successful only
in one case. However, this must be kept secret in the interest of an industrial
enterprise (9d,e).
In order to be able to carry out
such comprehensive tests which involved many people in the cellar of the
Geographic Institute of the Free University where we were "uninvited
guests" for a certain time, we had converted a little used bicycle cellar
into a mouse lab. The room was ventilated sufficiently, and on tables we installed
1000 plastic dishes and wire covers and drinking troughs (sponsored by BASF).
The "mouse cellar" converted
from a former bicycle cellar had been equipped with good ventilation. However,
the construction department of the Free University had placed the outlet for
waste air next to the large rhododendron shrub to the right of the main
entrance of the Geographic Institute. At a departmental meeting the director of
the Geographic Institute complained about the mouse odour which, depending on
the wind, was noticeable more or less, but all he got as a reaction were grins,
and no change was made.
For easier understanding, here are a
few remarks about the "accommodation" of the biochemical department
during the time before we moved into our own buildings in Lichterfelde-Süd, Ostpreussendamm,
and in Lichterfelde, Limonenstrasse. The department was first a sub-tenant at
the Pharmaceutical Institute in Berlin-Dahlem, was then extended to occupy (by
force) a few rooms in the cellar of the Geographic Institute in Berlin
Steglitz. The geographer called from
Only after the renovation of the
building of the Institute in Lichterfelde Süd was completed, did things become
normal. In Lichterfelde-Süd, we were able to work chemically, but another solution still had to be found for purely biochemical issues, namely with the
second building for the Institute at Limonenstrasse in Berlin-Lichterfelde
(1950-1969).
Banishment of Mrs M.Taneja,
BSc.,MSc.(
The author would like to point to
the long-term cooperation of Ms. Taneja
on the projects "Cannibalism", "Investigations of the vitamin B6
complex and its anti-vitamins in the intermediary metabolism", and
"Cannibalism and 4-deoxy-pyridoxine". These were some of the
above-mentioned 20 series of trials characterised by the example of Table 1,
especially experiments to characterise the interference of 4-deoxy-pyridoxine (I) in greater detail and, if possible,
to shed some light on it. In addition, Ms. Taneja compiled an almost complete
collection of literature on the topic "Cannibalism", procured the
most important original works and - where necessary - organised and financed
translations.
Ms. Taneja is a member of a high Indian caste and had
substantial funds[7]. Starting in 1964, she
worked as an unpaid freelancer at the Biochemical Institute of the Free University
of Berlin, first out of interest in scientific issues and later - with the
encouragement of the author - with the
objective of writing a thesis to be submitted later to her home university in
Ms. Taneja herself did not take any
legal steps. She was deeply hurt and left the "University". As an
Indian lady and member of a high caste she avoided any kind of dispute. Such an
act would have been beneath contempt for her. This is why the scientific experiments
to throw light on the effect of I
could not be completed in


Regarding Topic 2:
According to more recent investigations, the vitamins C and E are
regarded as "transport vessels” for hydride ions (16): Plate 5 and 7 (23).
Worth mentioning are discussions of
the author with Professors Dr. H. Remy and Dr. H.H. Schlubach, Chemical State
Institute of the University of Hamburg which, according to the records from the
lab diaries, took place in 1940 and 1946 on the topic "Existence,
stabilisation, detection and benefit of negatively charged hydrogen ions".
The questions raised both in 1940 and later were: Can hydride ions be used to
intercept radicals? Where do they occur in nature? Where should they be
expected? Stabilisation? Are hydride ions better radical scavengers than the
vitamin C complex?
These questions were not answered
until 40 to 50 years later by the works of American scientists which are
described in greater detail in (16). The detection and measurements of the
stability of hydride ions in solutions were carried out in the 1990s with the
aid of the following three methods:
1) Method H-NMR = H-Nuclear Magnetic Resonance Spectroscopy
2) Method ISE =
Ion Selective Electrode Potentiometry
3) Method XRD =
Wide Angle X-Ray Diffraction Spectroscopy
Accordingly, stable hydride ions exist in aqueous solutions which were detected
qualitatively with all three methods and quantitatively with method 2: under
suitable conditions, they are detectable for
several weeks.
In the opinion of the author, the therapy with fruit and vegetable
juices successfully used by
In closing, reference is made to
additional investigations of the author connected with vitamins:
„Anstieg des Kollagen- und des Vitamin-C-Gehaltes in Humanplazenten“ (17),
„Zur Chemie von Magnesium-L-ascorbyl-2-phosphat, C6H6O9PMg3/2“ (18),
„Vitamin-E-Komplex und Vitamin E-Nikotinat (d,l-α-Tocopherol-nikotinat) und deren durchblutungsfördernde Wirkung sowie deren vasodilativer Effekt auf die Haut“ (19a,b).
Haut- und Massageöl (Castanum), based on Japanese Olbas Oil, developped in cooperation with Prof. Köhnlechner (21): cf Photos there.
On the occasion of the meeting of
Nobel prize winners in Lindau in the late sixties, the author had the
opportunity to discuss his topic of special interest, ascorbic acid, with Prof.
Dr. Lines C. Pauling (22), and to show him some Plates. For details, see
quotation (23).
The reader may have noticed that
different manners of writing were used for ascorbic acid in the Plates 2, 3,
and 6 to 8, depending on what required special explanation. The principle of
speech to use the simplest expression possible also applies to chemical
formulae. These are a kind of projection
of spatial models (STUART or others) on paper, i.e. the formulae often do
not show the position of the atoms vis-à-vis each other correctly. This is
particularly evident when comparing the O arrangement of the I formulae in said
Plates. Another example: To better show the asymmetry caused by C4
and C5, the I formulae shown in Plates 6 and 7 were rotated
accordingly (C4 to C6 are positioned vertically).
For reasons of originality, it may
also be desirable to stick to the original manner of writing used in a
publication; for example, this applies to the Plates 6 to 8 used as a kind of
basis for discussion (23).
APPENDIX with Plates 2 to 8
Plate 2:
Photocopy of a publication describing the results
of the application of the vitamin C complex as stabiliser including the overall
vitamin C determination

Alimenta redaction
board permitted further publication of this paper already 1980
Plate 3:
Connections in the form of formulae between
important monosaccharides of the C6 series and derivatives
formulated after their occurrence (not according to Fischer's rules, cf. also
the formula scheme to IIa, IIb and IIc in Plate 1).
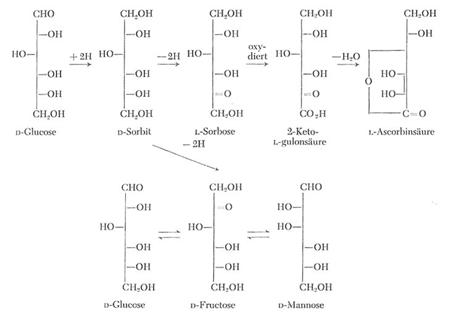
The structural
chemical correlation between D-glucose and L-ascorbic acid [IIa] is of primary
interest here. The II a pre-stage 2-keto-L-gulonic acid (Plate 1) is generated
by L-sorbose oxidation (preparatively directly through catalytic oxidation with
oxygen from the air at the platinum contact). See also text under Plate 1 and
references (20,8b).
On the next page follows Plate 4 on the topic
"Existence of pangaminic acid alias vitamin B15?"
A few comments in advance:
Several companies that supplied
pangaminic acid or vitamin B15, respectively, at the time, were
informed by the author of the negative findings on this topic, for example
through published articles (15). Reaction: None. Only one company answered to
the effect: "Dear Professor, you may be right with what you say, but I am
sure that we will continue to sell pangaminic acid for many years" (1985).
Many thanks for the valuable
contribution of Mr. Gerhard Quelle on the project vitamin B15 and
pangaminic acid. Mr. Quelle carried out the comprehensive literature studies
mentioned above.
Plate 5:
copy of publikation, citied in (16); see also Plate 7 and 6 (23)
Nutritional Supplement by Hydrid Ions acting as
Antioxidants and Hydrid Ions and H Atoms as "Energy Currency" for
Living Systems
by
We know for a long time already that we can
enrich our nutrition by fresh fruit- and vegetable juices. The American
nutrition specialist Dr. Norman W. Walker[8] was one the first to point out the
therapeutical value of the mentioned juices. Since 1930 he applied this
knowledge succesfully to his patients and himself for many years (1),
even thouth he could not give a scientifically proven explanation. Dr.
Walker reached the age of 116 in good health.
Today we know – 70 years later – that anyone
consuming a certain amount of fresh and raw plant
or animal food of good quality will automatically obtain a significant quantity
of hydrid-ions which are more stable in aqueous solutions than expected.
Firstly found and analyzed in the nineties by new physical methods (2).
The content of negative hydrid-ions in nutritions is drastically reduced by
heating, prolonged exposure to air or storage, by milling ecc. Negative
hydrid-ions are antioxidants (=reducing agents, electron donors). They can
easily neutralize radicals leaving only water as "ash": Radical
scavenger effect of hydrid ions.
Early in the 20th century Professor
K. Langmuir, one of the fathers of inorganic chemistry, proved the existence of
negative loaded hydrogen-ions and hydrogen atoms in H2-gas at higher
temperatures: H2→ H+ + H- and
H2 →H + H (3)
Already in 1940, the author (4) was discussing
the subject with two of his professors during his studies of chemistry in
In July 62 the author (5) held a lecture with
the subject "possible protection of cancer by radical scavenger" at
the LIONS-Club-Berlin, by invitation of the lawyer Ludwig, Prof. Dr.
Friedeboldt, General Consulate W. Böttger. The main point being the postulated
hydrid ions. The behaviour of hydrid ions in aqueous solutions was subject of a
lecture held in October 1962 in Wädenswil (6)
It was only many years later, namely in the
1990s, that the existence of stable hydrid ions in aqueous systems could be
proven. There are three methods (2), (7):
H-Nuclear
Magnetic Resonance Spectroscopy (H1-NMR),
Ion Selective
Electrode Potentiometry (ISE), and
Wide Angle X-Ray Diffraction Spectroscopy (XRD).
C.J. Stephanson and co-worker (2) stated:
"As significant and substancial as the XRD- and ISE-Analysis are, the
NMR-analysis is without a doubt the most definitive proof that the stabile
hydrid ion can exist in an aqueous environment, even over an extended
time." The ISE methode allows to determine quantitatively
hydrid-ions for several weeks under suitable conditions (2).
Often the hydrid ions are so to speak
"hidden", loosely bound in structure hydrides or organic compounds
like NADH, flavonoides, vitamin C- and vitamine E-complexes. Vitamine E
for instance acts as "transport vessel" for hydrid- ions. Hydrid-ions
[ H-] respectively H-atoms [H = H+ + ө ] can be
seen as "energy currency" of living systems.
For better understanding the author found the following text very
interesting
"The negative
hydrogen ion (H--ion) is a powerful, primitive primary antioxydant
found in all raw, unprocessed, untreated foods (plant and animal) and in many
"wild" unprocessed water supplies (glacial runoff water, high
altitude wells and springs, some deep wells, etc). It was the original
antioxydant for life forms on earth, and is likely the single most optimal antioxydant
for life form.
By the 1990's it
became apparent the H-ion is ubiquitous even in life forms on earth, and
essential to certain key biochemical reactins related to the citric acid cycle
(KREBS-cycle) in living organisms. By the late 1990's, it became obvious that
several common antioxydants found in plants and animals (Vit.-E-complex among
them) function as an antioxydant by acting as a transport vessel for the H—ion,
donating it at the right time within living systems to neutralize any of
several species of hydrogen free radicals (oxydizing radicals), also known as
reactive oxygen species (ROS) occurring in tissues or fluids in or around the
cells. The binary pair, NAD, NADH, is an excellent example of these facts. NADH
is well known as a potential energy carrier in living systems, and to
play a key part in the energy currency of most cells of many life forms,
including those of humans, while NAD is the low energy combustion
product." http://www.rawpaleodiet.org/h-ion-index.html
(October 2001):
The above mentioned authors C.J. Stephanson,
C.F. Duffy, A.M. Stephanson, and G.P. Flanagan (2) explained: "Since
the human body is majority water, the ability to know definitively that a
hydrid ion exists and is stable has significant importance to understanding
biochemical mechanisms of reactions in the body . Up until recently, the
NAD+ to NADH conversion was thought to mechanistically be carried
out through the transfer of a hydrogen proton and two separate electrons. The
present theories understand the mechanism to be related to a hydride transfer
rather than a hydrogen atom(8). Knowing that the hydride ion does exist in
the aqueous environment could allow further investigation into the biochemical
reaction mechanisms"
Below, some details about the two researchers
and theresults of their research that have decisively influenced the areas
under discussion: 1) Prof Dr Patrick Flanagan,
To 1): "Research on water and
longevity" was an important field of work for Flanagan, especially
"Studying the Hunza water and other therapeutic waters". The waters
of the Hunza have blessed the inhabitants of the Hunza mountain valley in
The water of the Hunza contain no dissolved
minerals in ionic form, but rather it is rich in extremely small mineral
particles (colloids) and rich in hydride ions, only rarely found in most water.
Flanagan describes Hunza water as "structured" (a sort of
"liquid crystal"). The surface tension of Hunza water roughly equals
that of our body fluids. Flanagan attempted to produce a "copy" of
Hunza water, eg by adding appropriate colloids and seperately produced hydride
ions (so-called microcluster colloids as Crystal Energy).
Flanagan also discovered hydride ions in some
other therapeutic waters, for example in the water at
To 2): Prof Walker (1) reported in great detail
on his long years of experience in the works cited. Part of his exposition -
above all about the state of knowledge on the subject of "intermediary
metabolism" - must, however, be considered outdated. But his basic idea on
the therapeutic value of fruit and vegetable juices remains unaffected by this.
The important thing is that the more recent research noted above (2) has
provided experimental proof. In his papers,
During his 60 years of work in the fields of
nutrition and health he proved that a long life and good health can go hand in
hand: Good health is independent of age. Dr Walker's interest in how to lead a
healthy life began in
References to (16):
(1) Norman W. Walker, "Frische Frucht- und Gemüsesäfte", Goldmann-Verlag, München, 1995; title of the original: "Fresh Vegetable and Fruit Juices", Norwalk Press, Pressot, USA; "Become Younger", USA, German translation: "Auch Sie können jünger werden", Mosaik bei W. Goldmann-Verlag, München, 1993; "Strahlende Gesundheit", ibid
(2)
C.J. Stephanson, G.P. Flanagan, C.Duffy:
"Evidence of Stable Hydrides in an Aqueous Environment, using modern
Analytical Techniques" J. Amer.Chem.Soc.2001, cited by C.J. Stephanson,
A.M. Stephanson und G.P. Flanagan: "An in vitro Cellular Analysis of
Radical Scavengings Efficacy of Silica Hydride on Hydroxyl, Superoxyde and
Singlet Oxygene, Reactive Oxygen Species., by Photosensitisation",
University of Minnesota, Minneapolis, MN; ibid(em) further literature
(3)
J.Langmuir, "Flames of Atomic
Hydrogen", Ind. Engng Chemistry 19 (6), 667-674, (1927)
(4) R.Riemschneider, H.Remy, H.H. Schlubach
Discussions in 1940 about the "Existence, stabilization, proof and use of negatively charged hydrogen ions"("Existenz, Stabilisierung, Nachweis und Nutzen von negativ geladenen Wasserstoff-Ionen.")
Author’s laboratory manual: Notes of talks on 15 Mar 40 and in May 40
with profs H Remy (Anorganic Chemistry,
Raised by the author
after a lecture by Prof Remy in Mar 40, the subject of "hydride ions"
was – at Prof Remy’s initiative – pursued under Prof Schlubach, the Head of
Organic Chemistry, State Institute of Chemistry, University of Hamburg: It was
above all the possible use of hydride ions as scavengers of free radicals that
made the stabilization and production of hydride ions interesting. Further
questions raised in this connection were: 1) can hydride ions actually scavenge
free radicals 2) where hydride ions occur naturally 3) where are they to be expected?
(5) R.Riemschneider
"Possible protection against cancer through free-radical
scavengers" ("Möglicher Schutz vor Krebs durch Radikalfänger").
Lecture given in LIONS Club, Berlin, in July 1962 at the invitation of attorney
Dr Ludwig (owner of the Sportpalast), Prof Dr F Friedebold (director of the
Oskar Helene Heim), Wolfgang Böttger (Consul General of Haiti) all of Berlin.
(6) Riemschneider (Lecturer),
E.B. Grabitz
„Theorethical
considerations on the behaviour of hydride ions in aqueous solutions: two
theories including mechanism and kinetics"("Theoretische
Betrachtungen über das Verhalten von negativen H-Ionen in wässrigen Lösungen: 2
Theorien incl Mechanismus und Kinetik") Lecture, held in October 1962, at
WIDMER AG;
(7) NMR: R.K. Harris, Hydrides
in NMR. View,
XRD:
Siemens D 500 Diffractometer, 2,2kW, Flanagan Technologies, Inc.,
(8)
R.W. Nagorski, J.P. Richard
Mechanistic Imperatives for Enzymatic Catalysis of Intramol. Transfer of a Hydrid Ion, J. Amer. Chem.Soc. 118 (31), 7432 bis 7433 (1996).
Plate 5 end.
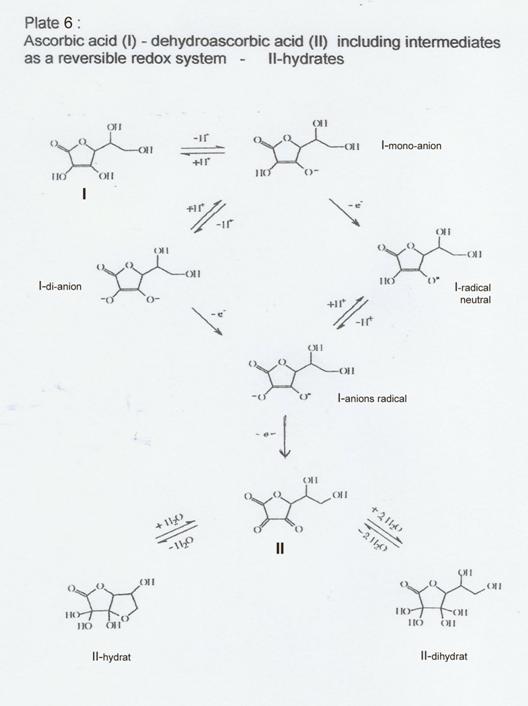
Plate 7:
„Freisetzung eines Hydrid-Ions“ durch Reaktion von I zu II (formal)
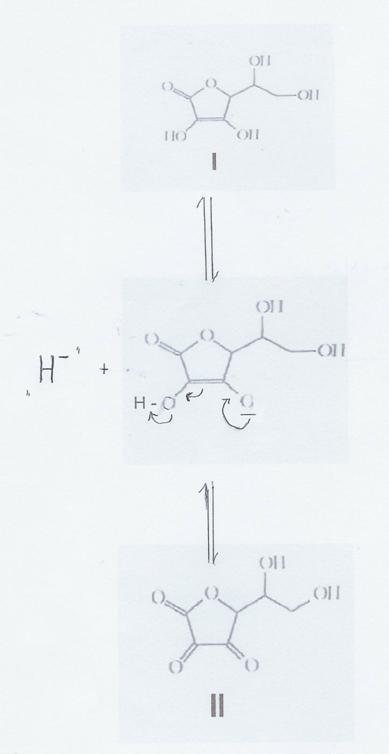
Plate 8:
Stereoisomeric forms of ascorbic acid (I); see ref. (23)
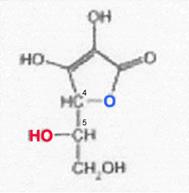
![]()
L-I D-I
![]()
L-iso-I D-iso-I
All of the I-stereoisomers shown are optically active: C4 and C5 are centres of asymmetry.
Because of the enzyme specificity, only the L-I has vitamin character among the I-isomers listed; D-I, L-iso-I and Di-iso-I are inactive. The low activity attributed to the isomer last mentioned could not be confirmed when working with the purest D-iso-I (free of L-I).
Regarding the definition of D and L, see ref. (20).
APPENDIX end.
BIBLIOGRAPHY:
(1) R. Riemschneider
„Vitamines and Antivitamines“ (Vitamine und Antivitamine)
(2) R. Riemschneider
„Vitamine und Antivitamine“
Vortrag, gehalten in einem Kolloquium des Hygienischen Institutes der Universität Jena im Januar 1945, im Zusammenhang mit dem in Kürze stattfindenden Rigorosum; Vortragsdauer 70 Minuten. Vorsitz: Prof. Dr. H. Schloßberger, Institutsdirektor. Anwesende: Prof. Dr. Bredereck, Prof. Dr. Brintzinger, Dr. Siebenmarck (Leipzig),
Zum Inhalt der Vorträge
1. Gegebene eigene Definition des Vitamin-Begriffes: Bei Vitaminen bzw. bei den Vitamin-Komplexen handelt es sich um organische Verbindungen, die 1) in kleinen Mengen mit der Nahrung aufgenommen werden müssen, die 2) der betreffende Organismus nicht selbst herstellen kann und die 3) aktiv in den Stoffwechsel eingreifen.
2. Man sollte von einem Vitamin-Komplex sprechen, wenn es sich um mehrere, in Wechselwirkung zueinander stehende Verbindungen handelt; zum Beispiel besteht der Vitamin C-Komplex aus L-Ascorbinsäure und Dehydroascorbinsäure [IIa/IIb]. Diese Betrachtungsweise spielt bei der Bestimmung des Vitamingehaltes in Naturstoffen eine entscheidende Rolle, z.B. (3a).
3. Wenn man von einem Vitamin-Komplex spricht, muß immer der zugehörige Organismus genannt werden: Der Vitamin C-Komplex IIa/IIb ist z.B. nur für den Menschen, einige Menschenaffen, Meerschweinchen und einige Vögel Vitamin. Die meisten Organismen können Vitamin C selbst synthetisieren.
4. Liste der bekannten Vitamin-Komplexe mit Formeln, eingeteilt in Wasser-
und fettlösl. Vitamine gemäß
Literatur.
5. Die wichtigsten Antivitamine sind diskutiert worden.
(3a) R. Riemschneider, E. Hausmann
Bestimmung von L-Ascorbinsäure und Dehydroascorbinsäure [IIa/IIb] nebeneinander (Gesamtvitamin-C-Bestimmung),
Laborberichte Januar 1945, 14 Seiten; vgl. auch spätere Veröffentlichungen und Anwendungen (10a-c, 10h), hier im Aufsatz besonders Tafel 2 (ANHANG).
(3b) R. Riemschneider
„Der Vitamin-B6-Komplex
[Pyridoxol, Pyridoxamin, Pyridoxal ] im Intermediären Stoffwechsel“,
Vortrag, gehalten im Dezember 1949 im internen Kolloquium des Physiolog.-chem. Instituts der Humboldt-Universität Berlin. Manuskript November 1949, 11 Seiten mit 5 Tafeln und 4 Tabellen; später Teil der Vorlesung „Intermediärer Stoffwechsel“: PROJEKT XXVI (8a); B6-Formeln in refs (9b)
(3c) R. Riemschneider
„4-Desoxy-pyridoxin (I)“
Vortrag gehalten wie vorstehend im Dezember 1949 – nach Arbeiten von W.M. OTT, Proc.Soc.Exptl.Biol.Med. 61, 125 (1946), 66, 215 (1947).
(4) http://ladywildlife.com/animal/cannibalisminanimals.html , 2003
(5a) R.
Riemschneider, H.-J. Hein
4-Desoxy-pyridoxin (I) als Kannibalismus-Auslöser bei trächtigen Nagern,
Laborbericht, 1957, 34 Seiten aus der Abteilung Biochemie der Freien Universität Berlin
(5b) R.
Riemschneider
Cannibalism by
administration of anti-B6 vitamins such as 4-deoxy-pyridoxin.
Methods for rodent control (Kannibalimus
durch Gabe von Anti-B6-Vitaminen wie z.B. 4-Desoxy-pyridoxin. Verfahren
zur Nagerbekämpfung).
Patent application in
(6) R. Riemschneider, H.-J.
Hein, H. Bode
Experiments
on influencing the gender ratio of newborn mammals before and during copulation
in 5 series of experiments with 5000 mice each (1000 ♂ : 4000 ♀) in plasticcages by keeping the male mice at a
weight of approx. 20 g.
6 lab reports, 1955 - 62, 310 pages in total, prepared
by H.-J. Hein (diploma in chemistry) on microfilm.
The experiments were carried out in the years 1955 to
1962 in the Department of Biochemistry of The Free University of Berlin in a
few rooms of the Geographic Institute in Berlin-Steglitz, Grunewaldstrasse,
after an out-of-use bicycle cellar had been converted into a mouse lab.
At the time, the
large numbers of newborn mice were donated to the aquarium of the Zoological
Garden (director Dr. Schröder) to feed the reptiles.
(7) R.
Riemschneider, A. Suhr, H. Kahl
On the negative influence
of regular intact yeast (baker's yeast) given with the drinking water on the
potency of male mice (Über den negativen
Einfluß normaler intakter Hefe (Bäckerhefe) auf die Potenz von Mäuseböcken,
verabreicht im Trinkwasser).
Lab report June 1938,
15 pages. Abbreviated version published in 1938 in the students' magazine of
These orientation
experiments were carried out during the time from May 1937 to June 1938 with
the approval of the administration of the Matthias-Claudius-Gymnasium, financed
by the family of my school friend A. Suhr from the Wilhelm-Gymnasium. (Suhr
studied medicine and obtained his M.D. after the war). These orientation
experiments (which were not confirmed sufficiently for statistical purposes) were
four series of experiments with a total of 960 mice of which 140 were controls.
The ratio in the test series I and II was
♂ : ♀
= 1 : 3, and in the test series III and IV 1 : 4. Evaluation by the
number of newborn mice. Result: Potency inhibition approx. 20 %.
This report was
continued in Bulletins II to XII (1946-84), entitled "Aphrodisiaka": PROJ
XXII in (8a); cf. also (6).
(8a) R.Riemschneider
Re-reading - 66
years chemistry,
26 PROJECTS, over 1400 references (in preparation), here concerning: PROJ. XXIII, bzw. XXVI.
(8b) R.Riemschneider,
Material für biochemische Einführungsvorlesungen 1969 (1.Auflage), 74 Seiten;
Druck: W.Hilke KG, Berlin 30
(9a) R. Riemschneider
Forschungsvorhaben: Markierte B6- und Anti-B6-Vitamine und ihre Anwendung auf Untersuchungen des intermediären Stoffwechsels und der Reproduktion
Antrag vom 30.6.1960, gerichtet an die Deutsche Forschungsgemeinschaft, Bonn-Bad Godesberg.
(9b) R.
Riemschneider
Position of the
vitamin B6 complex (pyridoxol, pyridoxamine, pyridoxal) in
intermediary metabolism - B6 deficiency and tryptophan degradation (Stellung des Vitamin B6-Komplexes
(Pyridoxol, Pyridoxamin, Pyridoxal) im intermediären Stoffwechsel – B6-Mangel
and Tryptophan-Abbau)
Lecture held in June
1960 at the Colloquium of RIEDEL de HAEN. ms 10 p (unpublished); also cf. (3b)
Vitamin B6 (pyridoxine, adermin)
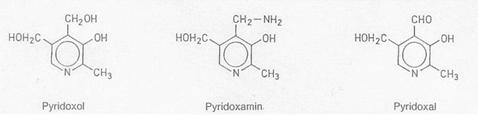
The term B6 comprises
the three substances pyridoxol, pyridoxamine and pyridoxal which may be
exploited by the organism in the same way. Pyridoxol is generally used for therapeutic
purposes, because it is stable against heat, alkaline and acidic compounds. The
really effective compound is pyridoxal-5-phosphate which is an essential
co-enzyme of transaminases and amino acid decarboxylases for the amino acid
metabolism.
pyridoxol: 2-Methyl-3-hydroxy-4,5-bis-(hydroxymethyl)-pyridine;
4-deoxy-pyridoxine,
actually: 4-deoxy-pyridoxol 2-Methyl-3-hydroxy-4-methyl-5-hydroxymethyl-pyridine.
(9c) R. Riemschneider and co-workers
Experiments
about the improvement of the 4-deoxy-pyridoxine (I) synthesis
Lab reports 1962/90 (excerpts).
Experiments carried
out at the Institute for Biochemistry of the Free University of Berlin and at
the Chemical Institute of the Federal University of Brazil S. Maria (UFSM),
(9d) R.
Riemschneider and co-workers
Synthese weiterer B6-Antivitamine (I-Analoge) und Prüfung auf Kannibalismus-erzeugende Wirkung bei trächtigen Mäusen, Ratten, Fischen
Laborberichte 1964/90 (sekretiert)
(9e) R. Riemschneider, M. Taneja and other co-workers
Versuchsprotokolle der „Kannibalismus-Versuche“ mit Anti-B6-Vitaminen (from 1964)
(sektretiert); cf. Table 1. There are protocols to each single animal of experimental series containing 500 mice each.
(10a) R. Riemschneider
Untersuchungen über den Vitamin
C-Gehalt von hitzesterilisierten und tiefgekühlten Kartoffeln,
Gutachten vom 1.10.1975 für die Firma FKF, Berlin 48, 50 Seiten (vermittelt durch einen ehemaligen Miarbeiter Dipl.Chem. Manfred Miehe, später Patent-Anwalt)
(10b) R. Riemschneider, R. Pereyron-Mocellin
Determinação de Vitamina C como critério de Caracterização de Alimentos Esterilizados através de Aquecimento,
Manuskript von August 1975, 21
Seiten (in portugiesischer Sprache); veröffentlicht in Revista Centro Ciencias
Rurais, S.Maria 6 (2), 1976.
(10c) R. Riemschneider, M.Z. Abedin, R. Pereyron-Mocellin
Mitt. I: Qualitäts- und Stabilitätsprüfung hitzekonservierter Nahrungsmittel unter Verwendung von Vitamin C als Kriterium,
alimenta 15, 171 – 174 (1976)
(10d) R. Riemschneider
Mitt. II: Kartoffeln auf dem Prüfstand – Mehr Vitamin C in hitzekonservierten oder in tiefgefrorenen Kartoffeln?
Betriebsverpflegung Heft 5, Seite 13 – 14 (1976)
(10e) R.
Riemschneider
Mitt. III: „L-Ascorbinsäure als
«Sauerstoff-Entferner» mit dem Ziel der Stabilisierung von Konserven und
pharmazeutischen Lösungen (Bulkware)“,
Vortrag vom 15.6.1976 vor einem
Gremium und Gästen der Firma FKF, Berlin 48; (nicht zur Veröffentlichung
bestimmt); vgl. auch (11).
(10f) R. Riemschneider, M.Z. Abedin
Mitt. IV a: Antwort auf die Lesebriefe der Hansa Fertigprodukte GmbH und des Deutschen Tiefkühlinstituts e.V. in Betriebsverpflegung vom August 1976, Betriebsverpflegung 1976, Heft 7, Seite 22 – 23
(10g) R.
Riemschneider
Mitt. IV b: Antworten auf die
Leserbriefe der Hansa Fertigmenue GmbH und des Deutschen Tiefkühlinstituts e.V.
in Betriebsverpflegung vom August und September 1976:
Manuskript 6 Seiten,
veröffentlicht in Betriebsverpflegung 1976, Heft 11
(10h) R. Riemschneider*),
R. Pereyron Mocellin**), M.Z. Abedin***)
Mitt. IV c: Determinação de
Vitamina C em batatas (Solanum tuberosum
L.) submetidas a dois processos de Industrialização, Rev. Centro Ciencias
Rurais, Santa Maria, Rio Grande do Sul, Brasilien, 7(3), 191 – 198 (1977)
*) Trabalho realizado no Instituto de
Biochímica da Universidade Livre de Berlím, Alemanha Ocidental.
Executor e Coordenador do
Convênio entre o Departamento de Química da Universidade Federal de Santa Maria
e o Instituto de Bioquímica da Universidade Livre de Berlím: Professor de
Bioquímica, Dr.rer.nat. (Doctor of natural, Science) ; Dr.h.c. (Diretor do
Instituto de Bioquímica da Universidade Livre de Berlím (West).
**) Auxiliar de Ensino do Departamento de
Química-UFSM.
***) Químico Diplomado na Universidade Livre de
Berlím (West)
(10i) R.
Riemschneider, M.Z. Abedin, I.K. Khan Ghouri
Mitt. V: Auf dem Prüfstand: Rindfleisch-Fertigprodukte, sinkender Vitamin B1-Gehalt bei kurzzeiterhitzten Rindfleisch-Fertiggerichten,
Betriebsverpflegung 1979, Heft 4, Seite 22
(10k) R. Riemschneider, I.K. Khan Ghouri, M.Z. Abedin
Hitzekonservierte Nahrungsmittel
Mitt. VI: Bestimmung und Stabilisierung von Thiamin in hitzekonserviertem Rindfleisch,
alimenta 18, 147 – 150 (1979)
(11) R. Riemschneider
Bull VII:
"Practical experience with and applications of L-ascorbic acid as an
oxygen scavenger according to Comm. Mitt. I - II" (Mitt. VII: „Praktische Erfahrungen and Anwendungen von L-Ascorbinsäure
als «Sauerstoff-Entferner» gemäß Mitt. I – III)
Lecture
held on August 15, 1975 at the Central Chemical Institute of the Federal University
Santa Maria,
Manuscript December
1980, 14 pages (unpublished); Manuscript in the Portuguese language published
in: Rev. Centro Ciencras Rurais, Santa Maria, Rio Grande do Sul, Brazil 9
(10), 120 – 125 (1979); also cf. (10a-e).
Annotation: The time-honoured method of preserving vegetables or fruit practised by
our grandparents (before 1930) is also based on the principle of oxygen removal
and a vitamin C reaction: The air is largely removed by heating, and the
bottles are sealed hermetically upon cooling. The remaining oxygen is
eliminated by the reaction with vitamin C. Any micro-organisms present have
been exterminated by heating.
(12a) R. Riemschneider, K. Hennig, Th. Wons
Keine
zellatmungsfördernde Wirkung aller nach der WARBURG-Methode geprüften
„Pangaminsäure“ – und „Pangaminsäure-Salze“
des Handels sowie auch der eigenen Syntheseprodukte [(1496a) in (8a)]
Laborberichte 1977 – 1982, 17 Seiten (unveröffentlicht); see (14)
(12b) R. Riemschneider, Th. Wons
Negative Versuche zur Herstellung von „Pangaminsäure (sog. Vit. B15)“ nach Angaben der Literatur,
Laborberichte 1977 – 1982, 20 Seiten (unveröffentlicht); see (14)
(12c) R. Riemschneider, Th. Wons, K. Hennig, G. Quelle
Comment on the
publication of A.K. Selezneva: Kletochnoe Dykhanie Norme Usloviyakh Gipoksii
1979, 108 – 109; Ed.. Khvatova, E.M. Gor’k.Med.Inst.
(13) R. Riemschneider, G. Quelle
Über das unentdeckte „Vitamin B-15“, die sog. „Pangaminsäure“,
Seifen, Öle, Fette, Wachse 109, 397 – 399, 440 – 444 (1983): 271 Zitate; vgl Tab. 2[9]
(14) R. Riemschneider, G. Quelle, Th. Wons, K. Hennig
Vitamin B15 – ein Wirkstoff, den es nicht gibt ? Keine zellatmungsfördernde Wirkung von „Pangaminsäure“ Zur Frage der Existenz der „Pangaminsäure“ alias „Vitamin B15“,
Kosmetik International 1983, Heft 4, Seite 11 – 12
(15) R. Riemschneider, G. Quelle
Existenz der „Pangaminsäure“ alias „Vitamin B15“?,
Fortschr. Med. 102, 339
– 341 (1984)
In der Bundesrepublik
Deutschland, einschließlich Berlin (West), und anderen europäischen Ländern
werden mindestens zehn pharmazeutische und drei homöopatische Präparate
vertrieben, die „Pangaminsäure“ oder „Vitamin B15“ enthalten sollen.
Gesichert sind jedoch weder die
Konstitution der „Pangaminsäure“ oder ihrer
Analoga noch ihre Existenz in der Natur oder in synthetischen
Präparaten. Die Interpretationen der meisten tierexperimentellen Untersuchungen
und medizinischen Anwendungen sind in Frage zu stellen. „Pangaminsäure“-
Verbindungen als „Vitamin B15“ zu bezeichnen ist wissenschaftlich
unhaltbar.; cf also Plate 4.
(16) R.
Riemschneider
Nutritional
Supplement by Hydrid Ions acting as Antioxidants – Hydrid Ions and H.Atoms as
“energy currency” for Living Systems,
Bi-Mouthly Journal of
BWW’s WHO’S WHO Society, Jan 2004, Irvine, Cal., USA Internet: http://www.bwwsociety.org/journal/html/hydrid.htm; Jan. 2004; copy in APPENDIX as Plate 5.
(17a) R. Riemschneider, M.Z. Abedin
Kollagen und Vitamin C-Gehalt von Humanplazenten.
Geburtsh. u. Frauenheilk. 38, 1066 – 1069 (1978)
Aus dem Institut für Biochemie der FU Berlin
(Geschäftsf. Direktor: Prof. Dr. Dr. h.c. R. Riemschneider). Please note: The function of a "managing
director" of a scientific board at FU is not comparable with an
"institute director" before 1969 (before introduction of the new
University Act), i.e. there is no contradiction to the above comments: As a
"comrade professor" one remains the team leader of a "tiny"
team of a scientific institution. Translation in footnote 9. [10]
(17b) R.
Riemschneider (Vortragender)
„Linearer Anstieg des Kollagen- und Gesamtvitamin C-Gehaltes in Humanplazenten“
Vortrag, gehalten im Dez 1976 im Kolloquium des Instituts für Biochemie der FU Berlin
(18) R. Riemschneider
“Magnesium-L-ascorbyl-2-phosphat C6H6O9PMg3/2 (IV)”
Vortrag, gehalten im Chemischen
Kolloquium des Instituts für Technische Chemie der Universität Jena im Januar
1945. Diskussionsleitung: Prof. Dr. H. Brintzinger, Laborberichte vom Januar
1945, 6 Seiten. Auszug aus dem Inhalt:
Beschreibung der Herstellung von IV und Analytik: UV von IV in 0,1 n HCl (1 :
50 000): 237 nm; 3 ml 0,3 %iger
IV-Lösung + 3 Tropfen Ammonmolybdat
in der Hitze: Gelbfärbung nach 5 Minuten blau. IV-Lösung mit Eisensulfat:
Braunfärbung
(19a) R.
Riemschneider
“Der Vitamin E-Komplex und das Vitamin-E-Nikotinat“
Vortrag, gehalten im Mai 1946 im Kolloquium des Pharmazeut. Instituts der Univ Jena,
Manuskript 17 Seiten (unveröffentlicht)
Die Texte der Vorträge vom Jan. 1945 und Mai 1946 sind hektographiert und nach den Vorträgen an die Anwesenden verteilt worden.
(19b) R.
Riemschneider, H. Vogt
Durchblutungsfördernde Wirkung und vasodilativer Effekt von Vitamin-E-nikotinat (V) auf die Haut,
Manuskript Dez 1947, 10 Seiten.
In Zusammenarbeit mit Dr. med. H. Vogt in Berlin konnten vasodilative Effekte von V auf der Oberfläche menschlicher Haut (ohne gleichzeitige Rötung wie bei Benzyl-nikotinat) beobachtet werden. Exakte Beweise stehen noch aus. Es fehlte uns eine gute Messmethode zur Registrierung von winzigen Temperaturdifferenzen und/oder zur Registrierung von Veränderungen der Wärmeleitung.
(20) R. Riemschneider
Stereochemistry of Heptites
http://www.bwwsociety.org/journal/html/heptites.htm 2007; there plate 4 and 5 with explanation of term “D” and “L”.
(21) M.
Köhnlechner, R. Riemschneider, W. Böttger
Präparat
auf Basis von japanischem Heilpflanzenöl (photo) – Vitamingehalt
Protokolle der Entwicklung 1983; cf.
SPECIAL PART, Re.: Topic 2.


R. Riemschneider, (left)
Prof. M. Köhnlechner (right)
As mentioned above, the author attended the Nobel Prize winner meeting
in Lindau described below and had occasion to speak with some of the lecturers,
Prof. Dr. Linus C. Pauling among them.
(22) Linus C. Pauling
Lecture on "L-ascorbic acid" on the occasion of the
meeting of Nobel Prize winners in Lindau in 1969.
Prof. Pauling reported
experiments where L ascorbic acid (I)
was administered in comparatively high daily dosages to persons for cancer
prevention; at the time, he was instantly criticised from a physiological point
of view. However, the significance of the hydride ions as radical scavengers
and the function of ascorbic acid (I)
and dehydroascorbic acid (II) as a
"transport vessel" for the hydride ions had not fully been recognised
over 35 years ago.
(23) R. Riemschneider
Diary records of discussions of the author with the lecturer, Prof.
Pauling on the topics:
![]()
![]()
a) Controversial cancer prevention by I II ; toxicological data
b) I II
as “transport vessels”, hydride ions ; (here Plates 6 and 7, formulated in 1966)
c) Stereochemistry of I (here Plate 8, formulated in 1960)
re
a) In
connection with the use of ascorbic acid in amounts which are considerably
above the necessary daily dosage, the author reported his own toxicological
experiments with rats. For I, the DL50
was determined to be about 12 g per kg of rat, i.e. for a humans of 60 kg, the
DL50 would be approx 0.72 kg, which is outside any discussion.
- In case of oral application of I to rats, our own experiments showed
that the concentrations of oxalate and therefore urea in the blood plasma
increased only after application of 20 mg I
per kg. The author had carried out these experiments in connection with
gerontological experiments also including I
(24a-c).
This discussion was not held in public, i.e. not after the lecture of Pauling,
so as not to encourage criticism of Pauling's experiments.
In the opinion of the author, it is still justified on the basis of today's
knowledge to take an additional 100 mg of I
per day if it is intended to exploit the radical scavenger effect of I for some time.
re
b) Because of his long involvement with
stability, function and detection of hydride ions, the author used Plates 6 and
7 to discuss the contexts formulated there and met with Pauling's approval,
just as for Plate 8 dealt with under c) below regarding theoretically possible I isomers.
Regarding the significance of hydride ions as radical scavengers, cf Plate 5 (16).
re
c) I-isomers and their "vitamin C character"
At
the time, the author intended to include the theoretically possible
stereoisomers of ascorbic acid formulated in Plate 8 in his work on cis-trans-asymmetry (25). The author asked
Pauling whether the latter had looked into the synthesis of the iso-isomers.
Both
scientists agreed that, owing to the specificity of the enzymes involved, only
L-ascorbic acid (in combination with the L-dehydroascorbic acid) will show
vitamin characteristics.
(24a) R. Riemschneider (Vortragender), O. Göhring, E. Frömming
„Intermediärer Stoffwechsel und Alter“
Mitt. II: Übersichtsreferat Fortsetzung, Vortrag vom 5.5.1956, gehalten im Kolloquium der Abteilung Biochemie der Freien Universität Berlin, Vortragsdauer: 120 Minuten
(24b) R. Riemschneider
Intermediärer Stoffwechsel und
Alter
Mitt. III: Altersabhängigkeit einiger Aminosäuren im Blut und „Normalisierung‘“ durch Cysteinzufuhr,
Z.Naturforschg. 16 b, 142 – 143 (1961)
(24c) R. Riemschneider, O. Göhring, E. Frömming
Intermediärer Stoffwechsel und Alter
Mitt. IV: Altersabhängigkeit des Gehaltes von Aminosäuren im Blut,
Z.Naturforschg. 16 b, 704 (1961)
(25) R.Riemschneider
STEREOCHEMISTRY: cis-trans asymmetrical compounds - axis-ring molecular aggregates http://www.bwwsociety.org/journal/html/stereochem
© 2006 The BWW Society/The Institute for the Advancement of Positive Global Solutions
Vol. VI NO.4 ISSN # 1544-5399 JULY/AUGUST 2006
Notes:
[1] For instance: Vitamin C is only a vitamin for humans, some anthropoide
apes, guinea-pigs and some birds, because of lacking a certain enzyme; all other living creatures can synthesize
ascorbic acid by themselves – as far as have been studied.
[2] The synthesis of I
and I-analogues has to undergo
several steps and is therefore rather complicated.
In this
respect, the author owes special thanks to Prof. Dr. Kinawi and Dr. R. Martin
who worked on the imrovement of the I
-synthesis and also I-analogues
thereof in
[3] Due to the university reform at the FU in
[4]
SEREX alias CELLRYL is a drug developed by the author which was used in
[5] Limits of yellow colouring in case of
SEREX taken into account in the quality standards of the product and agreed
with the Japanese customer.
[6] …decreased - with regard to the prolongation of live (when the number of cell division is regarded as defined)
[7] For example, more than 5000 guests were invited to Ms. Taneja's wedding
in
[8] He founded the "Norwalk Laboratory for Nutrition and Research"
in
[9] All cited papers had been studied in the original first and most of them had been translated for further research.
[10] Die Funktion eines auf Zeit gewählten „Geschäftsführenden Direktors“ einer wissenschaftlichen Einrichtung an der FU ist nicht vergleichbar mit einem „Institutsdirektor“ - ernannt vom Wissenschaftssenator mit Urkunde - ist kein Widerspruch zu oben gemachten Ausführungen3. Man ist und bleibt als solcher „Genosse-Professor-Arbeitsgruppen-eiter“ einer „winzigen“ Arbeitsgruppe, gehörend zu einer wissenschaftlichen Einrichtung, vgl. auch note 3.
For correspondence: Prof. Dr. Dr. R. Riemschneider, D-14001 Berlin,
Fach 1164, Germany,
e-Mail: rriemschneider@yahoo.de
[ BWW Society Home Page ]
© 2008 The Bibliotheque: World Wide Society
