The
Physiology of Transport Substances in the Blood (Sodium)
By Professor Marcel Uluitu, M.D. Ph.D.
Co-Authored by Diana Popa (Uluitu), M.D.
Department of Microbiology, Immunology
and Molecular Genetics
[Editor’s Note: This paper is presented as Part III of a
series of chapters from the new book “The Physiology of Transport Substances in
the Blood (Sodium)”; subsequent chapters will be featured in upcoming issues of
this Journal. This segment features Section III (of three sections) of Chapter Two,
Chapter Three and Chapter 4 (of six chapters)]
Chapter 2
(Section III):
The
Blood-Vascular Space
2.8. Na transport in the blood.
Na chemical activity in the blood is conditioned by
its interaction with local components.
It is carried mostly in a bound state. In the method presented one describes
the interaction of heparin anionic sites with Na and serotonin (5HT) cations.
The interaction occurs with different energy, according to the equations (217,
218)
![]() Na + heparin (H) = H-Na with an energy of interaction = E
Na + heparin (H) = H-Na with an energy of interaction = E![]()
5HT +
H = H-5HT interaction with energy E![]()
Initially, E![]()
![]() E
E![]() and in the mixture there are
and in the mixture there are
5HT+ Na + H = H-Na + 5HT. If Na is involved in
interactions with other substances, E will decrease until E![]()
![]() E
E![]() , and the equation will become
, and the equation will become
5HT + Na + H = H-5HT + Na . In this case Na chemical
activity is lower.
So the serum Na chemical activity refers to the interaction of 5HT with
H which is the reference for estimating
the activity of Na.
The determinations show that the chemical activity of
Na is lower than the reference value. This is consistent with normal nervous
excitability. The increase of the Na chemical activity is accompanied by
increased neuromuscular excitability: in rats by audiogenic seizures,
hypermotility, etc.In humans, they are expressed by behavior disorders of
constitutional type, EEG abnormalities, attention deficit disorders, disorders
of hemodynamic (see chapters of physiopathology). The interaction of Na with
proteins in the blood represents a barrier of energy in the way of Na motion as
a result of decrease of its chemical activity (218).
Na – proteins interaction in the blood has a
regulatory role for cation transport and also for mass transfer to endocellular
space of the excitable structure (218).
The chemical nature of the bonds discussed above, between Na with proteins and
the intensity of interaction are dependent on the variation of molecular
parameters of substances and of solvent. Na ligandation depends first on the
anionic and cationic composition of the solution (183) (Table 11), not only on
the concentration of ligand.
The ligandation depends also on extraprotein factors (solvent, Na) on molecular
protein factors(density and nature of anionic and cationic sites (Tb.11),
conformation, hydration, etc. Water, structured by hydrogen bond, is fixed on
the lateral, polarizable radicals and on the covalent bond (208, 160),it
stabilizes the protein molecule. Breking of the hydrogen bonds of water induces
protein denaturation and loss of Na (218).
Unpolarizable Na induces protein conformational
changes, increases the strength of anionic sites field (208, 166) and interacts
with the water dipole. The intensity of these influences is reduced in the
presence of other anions in solution and depends on the strength of the
cationic field of hydrated Na.The loss
of water molecules shortens ionic radius (Tab.9) (173, 126) and thus increases
the energy of interaction. The energy of interaction is thus variable, it falls
asymptotic to "0" from the center of ions towards periphery, defining
in this manner a number of crystalline rays (120) of the ions.The charge of Na
ions induces the increase of the energy of anionic sites of proteins
and diminishing rays of interaction
of the cation field. It interacts with all kinds of anions in blood (tb.11).
The degree of interaction depends on the polarizability anions. The Na
interaction with protein sites anion has decreased as a result of its chemical
activity and its capacity to react with other anions. Proteins are the most
important substances for the transport of Na. Proteins are amfoteric
substances, have great plasticity and
thereby enable the accommodation of various other ligands (191). Their capacity
of transport depends on their nativity (Table 17). Interacting with metals
they modify their charge by transferring
electrons from the metal. The intensity of interaction of anionic sites
of proteins with cations depends on other factors as well , such as (10) the number of coordination, the amino
acid composition, polarizability of anino groups (Ling cit.16).
Strength sites is also influenced by internal
neighboring groups, with variable polarity interacting through inductive
forces, with intensity dependent on the square of the distance between them
(120). The activity of proteins depends on the degree of dissociation of
anionic sites, according to the theory of multiple equilibrium (112): it
increases with the length of the polypeptide chain, with the pH of the solution
and the intensity of the electrostatic forces (4, 20, 191). The affinity of
proteins decreases by denaturation (233), the interest of coordination
involving prototropic groups of the aminoacids residue aspartame, glutamic, histidine,
treonine, cysteine, arginine. The charge density of the proteins ,
decisively influences the interaction
with CATIONS. Its decrease diminishes the
afinity for cations(166). The density of the charge of anionic
sites induces the formation of a cationic
cloud around them causing an unequal distribution of cations in the
neighborhood (161). The selection for monovalent cations (20) depends on the
charge density of the anionic electrostatic field and their degree of hydration
(20,181,170). The amount of the charge density of the proteins influences
inversely proportional the activity in solution of sodium (165.62).
The main issue is to decipher the mechanisms of Na
transport in the blood. The data presented so far do not suggest an
individualized transporter but present a general process in which blood proteins , or a multitude of anionic groups with which
Na can interact , are involved. The type of binding, as well , is not identified, being represented by
interactions that have been discussed at length. These are weak interactions
that have an influence mainly by their number and type , rather than by their
strength. Therefore the equilibrium of the three compartments does not depend only upon the gradient of the cation
but also on the distribution of anionic
groups between compartments. On the other hand the existence of olygoenergetic
bonds ( weaker than between 5HT and
heparin, taken as a reference value in the method used by us in this research )
enables us to underline some physicochemical properties of Na-proteins
interaction. Sodium involved in various complex interactions, is in equilibrium
with its free form, chemically active, as demonstrated by the method of
competition and it is compatible with the functional manifestations of the excitable
tissues. Increased chemical activity in humans and rats is accompanied by
cerebral hyperexcitability .There are no data available to explain, among other
things, the transfer mechanism of Na to the level of the excitable membranes, or the blocking of
ionic channels by using toxins.
Na interacts with any kind of anions from the blood (
table 11 )
2.9.
Method of determining the Na activity in blood serum. (Method of competition
5HT / Na for anionic sites of heparin)
2.9.1.
Principle of method.
For dynamic dialysis
the competition is determined between
Na cations and serotonin (5HT) for the anionic sites of polyanion
heparin (H).
Their affinity for heparin is different and can be
described by equations:
Eq. 1: Na
(mM)+ H(mg)![]() Na-H with
interaction energy = E
Na-H with
interaction energy = E![]()
Eq. 2:
5HT (![]() g)+H(mg )
g)+H(mg )![]() HT-H with
interaction energy = E
HT-H with
interaction energy = E![]()
Since E![]()
![]() E
E![]() we have:
we have:
Eq 3: 5HT
+ Na + H ![]() Na-H + 5HT Eq.3 is valid for any solution that contains
the active chemical Na, and interacts with other substances with E
Na-H + 5HT Eq.3 is valid for any solution that contains
the active chemical Na, and interacts with other substances with E![]()
![]() E
E![]() . In the presence of blood serum, the above equations are
written as follows:
. In the presence of blood serum, the above equations are
written as follows:
Eq.4: Native serum (sn) + H + 5HT ![]() 5HT-H + sn.
5HT-H + sn.
Eq.5: sn
+ Na + H + 5HT![]() Na -H + 5HT + sn. Na is of exogenous origin.
Na -H + 5HT + sn. Na is of exogenous origin.
Eq.6:
denatured serum (sd)+ 5HT + H![]() Na-H + 5HT. Na has endogenous blood origin. Dialysis allows
to obtain the necessary values to calculate and to identify the heparin anionic
sites.
Na-H + 5HT. Na has endogenous blood origin. Dialysis allows
to obtain the necessary values to calculate and to identify the heparin anionic
sites.
2.9.2.
Equipment.
2.9.2.1.
Spectrofluorometer.
Spectrofluorometer sensitive to ultraviolet, with double monochromator for the
separation of excitation radiation (![]() = 295 m
= 295 m![]() ) of fluorescence radiation (
) of fluorescence radiation (![]() = 340 m
= 340 m![]() ) for establishing the serotonin.
) for establishing the serotonin.
2.9.2.2. Dialysis cell. (Figure 4).
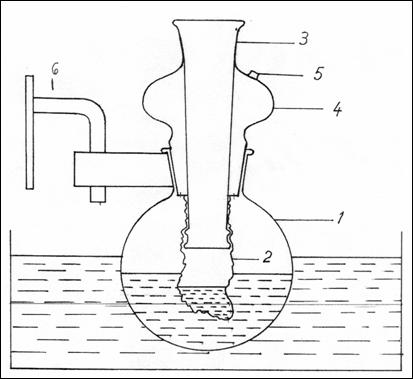
The dialysis cell is made of a balloon with round
bottom (1), with a polished neck in
which a glass tube is mounted(4), polished as well (3), with the outer end open , where
reactants are being introduced into the cellophane bag(2). This may be replaced
by a rubber stopper pierced by a glass tube.
The neck of the balloon has an orifice (5) where the solution of 0.25 M
sucrose is inserted in which the cellophane bag is immersed. The system is
equipped with a device for fixation on
the agitator (6).
2.9.2.3.Flamfotometer.
2.9.2.4. Preparation of the equipment.
A prerequisite is to avoid contact with ions of other
source or ion exchangers, detergents. For this purpose glassware is
abundantly flushed with double distilled water, dried and siliconed . Ion exchangers will not be used because fine particles of them
can remain in water and modify the reactivity of proteins and the interaction
with Na. The same steps are required in
the preparation of cellophane bags. Solutions are to be prepared only with
distilled water.
2.9.3.
Reagents: heparin, serotonin, sucrose, NaCl, water.
(1) heparin powder, G.M. = 10,000 - 20,000. It has anionic groups as SO![]() H, active at pH close to neutrality.
H, active at pH close to neutrality.
(2), serotonin (5-hydroxi-tryptamine = 5HT) as a serotonin-creatine-sulfate
solution of 1![]() g active substance in one
milliliter of sucrose solution of 0.25 M
g active substance in one
milliliter of sucrose solution of 0.25 M
(3) Sucrose p.a. 0.25 M as a medium of isotonic reaction.
(4) NaCl p.a.
(5) bi-distilled water.
2.9.4. Technical procedure.
Calculations are carried out with the dialysis cell thermostatically controlled
and stirred with a speed of 80 cycles / min. The characteristics
of the 5HT dialysis in the bag is
determined both in the absence and in the
presence of different concentrations of heparin (Figure 5, Table 14).
The rate of the liquid volumes : there are 2 ml reactants in the solution of
0.25 M sucrose in the bag and outside the bag there are 58 ml 0.25 M sucrose
solution in which the 5HT from the bag dialyzes , thus expressing the processes
that take place before dialyzation.
2.9.4.1. Characterization of the dialysis bags
The permeability of bags against heparin is to be checked. A spectrophotometric
measurement is to be made in the dialysis fluid outside the bag, in order to
see the color resulting from heparin with toluidine blue. Should heparin pass
through the pores of the cellophane bag, alteration of results can be
avoided by acetylating the bags by
dipping them into a mixture of acetic anhydride and pyridine until the reaction
with blue toluidine becomes negative, or else other bags can be tested.
2.9.4.2. Measurement of the dialysis coefficient
0.1704 ![]() M (60
M (60 ![]() g) serotonin active substance in 2 ml final volume of sucrose
solution 0.25 M are to be introduced in the cellophane bag. At the end of the
dialysis, outside the bag there will be a concentration of 5HT of 1
g) serotonin active substance in 2 ml final volume of sucrose
solution 0.25 M are to be introduced in the cellophane bag. At the end of the
dialysis, outside the bag there will be a concentration of 5HT of 1 ![]() g /ml, the increase of which is measured every 5 min. (tables 14, 15). Passing of 5HT outside the bag takes place under the law
of diffusion of Fick (103) described as :
g /ml, the increase of which is measured every 5 min. (tables 14, 15). Passing of 5HT outside the bag takes place under the law
of diffusion of Fick (103) described as :
Eq.
7 (C![]() - C
- C![]()
![]() ) / (C
) / (C![]() - C
- C![]()
![]() = e
= e![]() where :
where :
C - C = concentration rate at 5HT EVRY "t" =
5 min.
C - C = concentration 5HT rate at "t" = "0"
"D" = coefficient of diffusion.
"T" = time in minutes.
![]() = constant characteristics that include bag:
thickness, porosity, etc.
= constant characteristics that include bag:
thickness, porosity, etc.
![]() = 1 / v
= 1 / v![]() x 1 / v
x 1 / v![]()
One bag can be used several times after
washing with bidistiled water for 10 -15 minutes, using the same
"D". Comparative measurements allow the ![]() and
and ![]() to be automatically included
into the "D" value .
to be automatically included
into the "D" value .
The calculation of diffusion coefficient "D"
depending on the concentration of heparin (Table 15, Fig 5) and under the
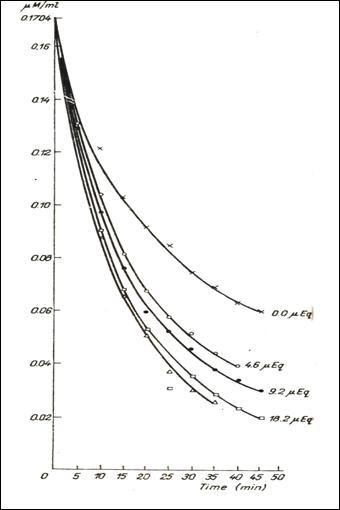
influence of Na (Table 16 and Figure 6).
Eq.
8 (C-C![]() )
)![]() / (C - C
/ (C - C![]() )
)![]() =e
=e![]() of Fick
of Fick![]() s law leads to the conclusion that the process
s law leads to the conclusion that the process
of diffusion depends only on the nature and physical-chemical status of the diffusible
substance, 5HT (18). "D" is used to calculate 5HT-free running ,
diffusible in the mixture of reactances in the bag.
Figure 5. Dynamics of the 5HT dialys in the bag , in the presence of variable quantities
of heparin and in the absence of it (232)
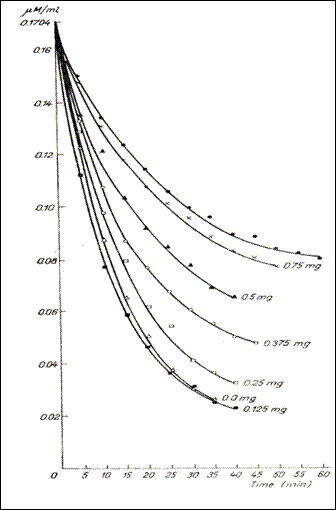
Figure 6.
Dynamics of the 5HT dialysis in the bag , in the presence of 0,5 mg of heparin
and of variable quantities of Na and in
the absence of it(232).
2.9.5. Determination Na/5HT competition for anionic
sites of heparin.
2.9.5.1. Determination of anionic sites of heparin.
Table 14. Diffusion coefficient (D)
depending on the concentration of heparin (232)
heparin 0 1.25
0.25 0.375 0,5 0 75
"D" 0 0624
0,0725 0,065 0,0593
0,06085 0,0561
Table 15.
Diffusion coefficient (D) in the presence of variable amounts of Na (232).
Na mEq/ml 0 4,5
9 18 32
D 0.0609 0,0608
0,063 0,0608 0,0614
Table 16. ![]() M anionic sites depending on the concentration of
heparin(232)
M anionic sites depending on the concentration of
heparin(232)
heparin mg/ml
0.175 0.25 0.375
0.5 0.75 1,o
![]() M anionic 0,0136
M anionic 0,0136![]() 0, 02
0, 02![]() 0 041
0 041![]() 0,0603
0,0603![]() 0,0747
0,0747![]() 0,0899
0,0899![]()
sites 0,004 0,0021
0,002 0.00505 0 012
0,009
1 mg heparin, 60 ![]() g serotonin, complex salt in 0.25 M sucrose, the final amount
of 2.0 ml , are being introduced into the dialysis bag. The bag is
immersed in the 58 ml dialysis solution,
sucrose 0.25 M. The dialysis cell thus
prepared is fixed on the stirring device (Fig 4) and introduced into the bath with a temperature
of 20
g serotonin, complex salt in 0.25 M sucrose, the final amount
of 2.0 ml , are being introduced into the dialysis bag. The bag is
immersed in the 58 ml dialysis solution,
sucrose 0.25 M. The dialysis cell thus
prepared is fixed on the stirring device (Fig 4) and introduced into the bath with a temperature
of 20![]()
![]() 0,1 C and stirred with a frequency of 80 cycle / min. At
baseline in the bag there are two forms of serotonin: one free, dialyzable and
other bonded, in interaction with the anionic sites of heparin. While 5HT free
running is difusing outside the bag , the
equilibrium between the bonded and the free forms left in the bag is
disturbed , initiating thus defixation processes (Fig. 6) which maintains the concentration rate of the free form. The reaction continues
until an equilibrium is reached between
the interaction energy 5HT-heparin and
the strength of dialysis induced by the
concentration rate (Fig 6). To determine the parameters of interaction ligand -
heparin (number of anionic sites of heparin and the association constant ligand - heparin) the free and bonded serotonin quantity is determined during
dialysis, at intervals of 5 minutes, until the equilibrium dialysis is reached
. The calculation of the two forms of 5HT is by Eq.9.
0,1 C and stirred with a frequency of 80 cycle / min. At
baseline in the bag there are two forms of serotonin: one free, dialyzable and
other bonded, in interaction with the anionic sites of heparin. While 5HT free
running is difusing outside the bag , the
equilibrium between the bonded and the free forms left in the bag is
disturbed , initiating thus defixation processes (Fig. 6) which maintains the concentration rate of the free form. The reaction continues
until an equilibrium is reached between
the interaction energy 5HT-heparin and
the strength of dialysis induced by the
concentration rate (Fig 6). To determine the parameters of interaction ligand -
heparin (number of anionic sites of heparin and the association constant ligand - heparin) the free and bonded serotonin quantity is determined during
dialysis, at intervals of 5 minutes, until the equilibrium dialysis is reached
. The calculation of the two forms of 5HT is by Eq.9.
Eq.9: dC![]() /dt = - DC
/dt = - DC![]() that:
that:
C![]() = total concentration of the ligand (free + bound) of the
bag every "t" = 5 min.
= total concentration of the ligand (free + bound) of the
bag every "t" = 5 min.
C![]() = concentration of the free ligand every t = 5 min.
= concentration of the free ligand every t = 5 min.
D = coefficient of diffusion of 5HT.
This
equation is not satisfactory because the time interval of 5 minutes is very short and gives large errors
especially when the absolute diffusion and the variation of concentrations
outside the bag are small (120) close to the equilibrium of dialysis, when
processes of defixation are small as compared to those of diffusion of ligand.
Therefore the form of successive approximation of eq. 9 is recommended.
Eq. 10: C![]() = (v
= (v![]() + v
+ v![]() ) /v
) /v![]() x C
x C![]() /1 –e
/1 –e![]() in which
in which
C![]() = concentration of the free ligand from the bag at "t" = 0
corresponding to each point of determination.
= concentration of the free ligand from the bag at "t" = 0
corresponding to each point of determination.
C![]() = 5HT concentration of dialysate every "t" = 5 min
= 5HT concentration of dialysate every "t" = 5 min
v![]() = volume of liquid in the bag
= volume of liquid in the bag
v![]() = volume of liquid outside the bag
= volume of liquid outside the bag
D = coefficient of diffusion.
The value of C![]() (the amount of serotonin fixed at t = 0)results from the
value of C
(the amount of serotonin fixed at t = 0)results from the
value of C![]() .
.
C![]() = amount of free 5HT every "t" with the equations:
= amount of free 5HT every "t" with the equations:
Eq.
11: C![]() = C
= C![]() - C
- C![]()
Eq.
12: C![]() = C
= C![]() - C
- C![]()
C![]() : is the initial amount of serotonine. Eq 10 allows
highlighting decomplexation processes that characterize multiple
equilibriums of the polyanion with small
ions by the graphical representation of the equation 13:
: is the initial amount of serotonine. Eq 10 allows
highlighting decomplexation processes that characterize multiple
equilibriums of the polyanion with small
ions by the graphical representation of the equation 13:
Eq.13 v![]() + v
+ v![]() / v
/ v![]() x C
x C![]() = f(1-e
= f(1-e![]() ) (Fig 7)
) (Fig 7)
Non linear graph indicates the presence of defixation
processes.
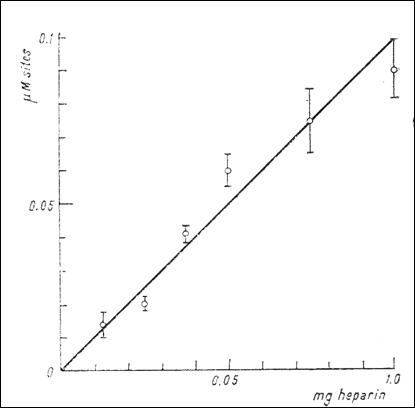
Figure 7. The dependence of the number of anionic sites with high affinity for
serotonin (μM) upon the concentration of heparin (mg) (232)
2.9.5.2.
The calculation of the association constant 5HT / heparin and of the number of
anionic sites
The equation of multiple equilibriums is used for this
purpose . (Eq 14).
Eq.14
: C![]() =
= ![]() (n
(n![]() K
K![]() -C
-C![]() e
e![]() / 1+K
/ 1+K![]() C
C![]() + e
+ e![]() ) which for a small number of sites can be written as Scatchard’s Eg (181),(Eq. 15)
) which for a small number of sites can be written as Scatchard’s Eg (181),(Eq. 15)
Eq.15
: C![]() /C
/C![]() = nK -KC
= nK -KC![]() where
where
n = number (M) anionic sites
K = constant association of complex heparin-5HT.
Information
is thus obtained about the influence of small cations that compete for 5HT
anionic sites of heparin, when they are free in solution and about the
interaction energy equal or bigger than
E2 (Fig. 7). (Tb.16) shows the number of anionic sites at different
concentrations of heparin and the existence of defixation processes.
2.9.5.3. Determination of the 5HT – Na competition.
Heparin (0.5 mg / ml) 5 HT (30 g / ml) and variable
amounts of NaCl in sucrose 0.25 M are introduced into the dislysis bag. Further
the above mentioned protocol is to be
observed. Fig. (6, 7 and GT 17) show the displacement of 5HT by Na+
in the heparin complex , the curves getting closer to those obtained for
determination of diffusion coefficient (Fig.5). In Figure 11 the semi
logarithmic strait line represents a
constant of exchange. The dependence of competitiveness processes Na+ / 5HT depending on the amount
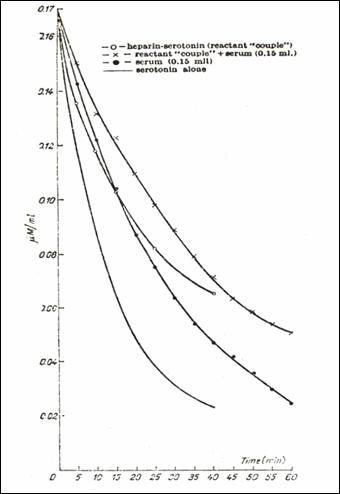
of active chemical Na+ can be noticed.
Figure 8. 5HT loss from the
dialysis bag under various influences (- ○ - the reactant couple alone; x the reactant couple
in the presence of native serum; - ● - native serum with no other
addition; - 5HT single reactant in the bag) (232)
2.9.5.4. The influence of native blood serum on the 5HT/heparin complex.
Collecting blood serum has to be made under the
conditions necessary to preserve the native state of macromolecular components
of the plasma. The blood is collected in tubes prepared as above. The blood is
collected through free flowing to avoid foaming. To store 15-20 minutes at room
temperature. Avoid low temperatures between 0![]() and 8
and 8![]() C because they induce severe changes to serum reactivity
macromolecules. It is centrifuged for 10 minutes at 2000 rpm. Blood serum is
separated in an other tube by free flowing, and the clot is discarded. The
content of protein, total electrolytes (by flam photometry) and chemical
activity of serum cations , of which 95% is Na+ , are determined in
the serum.
C because they induce severe changes to serum reactivity
macromolecules. It is centrifuged for 10 minutes at 2000 rpm. Blood serum is
separated in an other tube by free flowing, and the clot is discarded. The
content of protein, total electrolytes (by flam photometry) and chemical
activity of serum cations , of which 95% is Na+ , are determined in
the serum.
To determine the chemical activity of Na+ ,
0.15 ml serum, heparin and serotonin in the quantities mentioned above are to
be introduced into the cellophane bag. The data obtained from the dialysis of the serotonin in the bag are represented
graphically (fig.9)
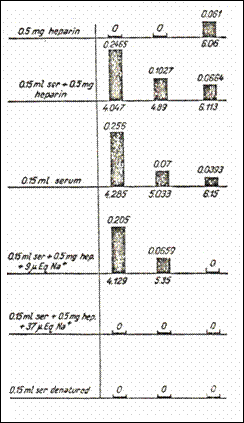
Table 17. Scatchard’ Eg.
parameters as: n =![]() M sites / ml solution and the PK of the sites in each group
(232)
M sites / ml solution and the PK of the sites in each group
(232)
reactants present n![]() n
n![]() n
n![]() pK
pK![]() pK
pK![]() pK
pK![]()
0.5 mg heparin (H) -
- 0 06 -
- 6,06
0, 15 ml native serum(SN) 0,256 0 07
0,0393 4,047 4,89 6,113
0, 15 ml (sn)+(H) 0,246 0103
0,0664 4,285 5,033 6,15
0, 15 ml (sn)+(H)+
9 ![]() Eq Na 0,205 0,066
- 4,129 5,35
-
Eq Na 0,205 0,066
- 4,129 5,35
-
0, 15 ml (sn)+(H)+
37 ![]() Eq Na
- - -
- - -
Eq Na
- - -
- - -
0, 15ml ser denatur. - - -
- - -
Figure 9.
- The influence of denaturizing upon the reaction 5HT-protein, as compared with
native blood serum ( native serum ● 0.15 ml; ○ denatured serum 0.15 ml, "x" 0.15
ml native serum, and Na 37 μEq) (232)
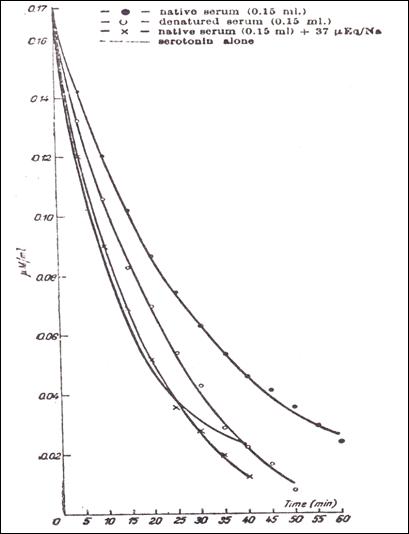
2.9.5.5. The influence of denatured blood serum upon the interaction
5HT/heparine.
The denaturizing of the serum is made by vigorously
shaking the tube , thus avoiding the addition of other chemicals. 0.15 ml of this serum is used instead of
native serum. Further , the above steps will be followed. The results of
dialysis 5HT (Fig.9) show that serotonin values are similar to those obtained
when the ligand is the only reagent in the bag, not interacting with heparin.
Figure
10. Representation of the number of sets of sites with high affinity for
serotonin. Every set is identified by pK under the image of each set in various combinations,
including native and denaturized blood serum (232).
2.9.5.6.The influence of exogenous Na on 5HT/heparină interaction
in the presence of native serum.
Variable amounts of NaCl solution are added into the bag containing native
serum . The curve of 5HT dialysis outside the bag is similar to that obtained
only with serotonin and to that where only denatured blood serum has been used.
(Fig.8, 9).
2.9.5.7. Determination and identification of the
number of anionic sites of heparin in the presence of blood serum.
This is done by graphical representation of Eq. (15)
by Scatchard (Fig 11, Tab 17).In the presence of denatured serum , heparin
does not interact with 5HT and a
straight line, parallel to the abscise ,is obtained(Fig.12). The subtraction
point by point of the two curves (Fig.12) can also be used.
Figure 11.The graphic calculation of the number of
anionic sites in the native and denatured
blood serum (232).
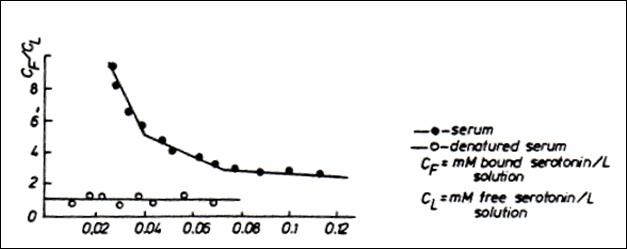
Figure12.Direct and semi logarithmic representation of decrease in the
number of anionic sites with affinity for 5Ht under the influence of variable
quantities of Na (232)
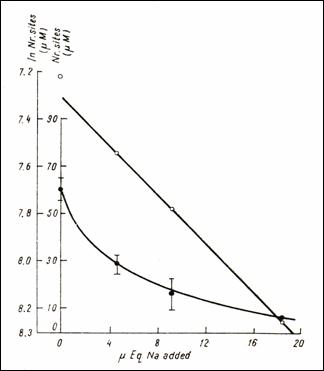
Figure 13.Quantitative binding of
5 HT ( Scatchard’s meth.) upon anionic sites in a mixture of serum and heparin
(○ couple reactant heparin + 5HT; ● couple reactant + native serum
0.15 ml ; CF = mM bound serotonin / l solution; CL = mM
free serotonin / l solution) (232)
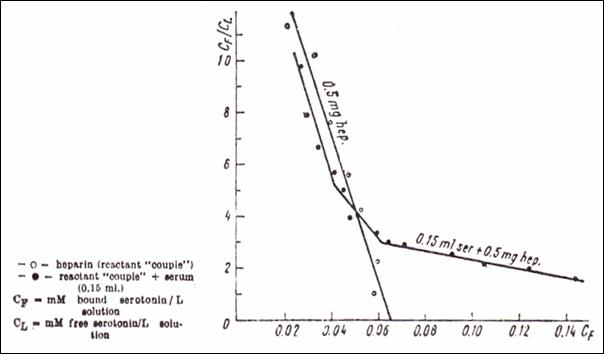
Figure
14. Graph equation CF = f(CL), where CF = 5HT
bonded on sites anionics; CL
= free 5HT (1) = native serum containing 10 mg% protein + serotonin (2) =
native serum containing 10 mg% proteins +
0.5 mg heparin+ 5HT (3) = native
serum with 10 mg% proteins + 0.5mg heparin + serotonin + 0.15 M NaCl (4) =
difference point by point between curve 2 and curve 1 representing μM
serotonin fixed on the anionic sites in the environment. (232).
In
conclusion, the method allows the determination of plasma Na+
activity and its interaction with proteins , as related to the reference value
of the interaction of 5HT with the heparin polyanion.
Chapter 3:
Extra
Cellular Matrix (ECM)
3.1.
General.
The cells of any tissue are held together by occlusive
junctions, which anchor the cytoskeleton on
the fundamental substance , the extra cellular matrix. The distribution
of the fundamental substance is uneven. In some tissues it is hard to identify
, like in epithelial tissues, poorly represented in the brain and spinal cord
and in very large quantities in bone and connective tissue (33, 66, 73, 111).
The matrix is composed of a variety of proteins and polysaccharides locally secreted and assembled into a network
in close contact with the surface of the producing cells. With all this variety
of distribution and structure, there is communication between all its
departments and they, remarkably maintain the essential aspects of the tissue
of origin (92, 171). Quantitative and structural variations of the matrix satisfy the requirements of the
function of each tissue ( calcified forms in bones, transparent ones in the
cornea, etc.). The pericellular matrix , rich in carbohydrates , is organized
as a network.
The matrix and the cytoskeleton are intimately
associated (116). Macromolecules from the matrix, in their turn , orientate the
cytoskeleton. In the area of separation between an epithelium and the
connective tissue (5),the matrix structures intself as a thin and hard baseline membrane , which
plays an important role in controlling the cellular compartment, in the development
, proliferation, shape and function of
the cell (55).
3.2.
The role of ECM.
First of all , it
physically stabilizes the parenchyma tissue, which it is part of .It has
multiple biochemical , informational , physical-chemical , metabolical etc.
influences. It maintains a constant character of cellular environment and
protects the parenchyma from variations in the blood composition , temporarily stores substances
until they are eliminated or included into the cell function. It mediates and
modulates exchanges of substance between the vascular and endocelular spaces :
electrolytes, fatty acids, oxygen, carbon dioxide, etc. It modulates the
activity of (140) of the compounds from cell environment, optimizing the intake
of substances in the cell. It has structures which analyze the composition of
intercellular matrix and of the
endocellular medium. It participates in the direct intercellular
communication. The matrix releases soluble factors under cell influences, with
response from the cells themselves. There are complex relations of
interdependence between the parenchyma cells and ECM : mechanical, biochemical,
information embryo genetic, immunological, transportation, etc. Between the
cells and the components of ECM there are connections of adherence and
molecular interaction.
3.3. The chemical composition of ECM. (156).
ECM macromolecules belong to two classes of substances: (1) glycozaminoglycans
(gag), which are made of free polysaccharide chains and (2) Proteoglycans,
composed of five groups: glycoproteins, collagen, elastin, fibronectine and
integrins.
(1) Glycozaminoglycans (mucopolyzaharid) consist of unbranched chains of
repeated units of aminosugers: ![]() -acetylglucozamin, acetylgalactozamin mostly sulfated and
associated with other types of sugar, uronic acids (iduronate and glicuroni).
The sulphate, carboxylic and hydroxyl groups in their composition confer upon
them an important electronegative charge with an important functional role.
They occupy large spaces and are very hydrophilic; they define hydrated and
porous gels in which the cells are enclavated. Their negative charges attract
clouds of osmotically active Na+ and large quantities of water. The
matrix (140) has great inflation pressure. The porous gels allow the diffusion
of hydro soluble molecules according to their size and electrical charge, such
as hormones, ions, catabolites, etc. There are four groups of special gags by
the composition of sugars, type of connection between them, place and number of
sulfate groups: hialuronan, condroitin, dermatan sulfate, heparan sulfate
cheratan sulfate, heparin. Hialuronan, hialuronic acid locally synthesized
conditions tissue swelling especially in saline medium (62).
-acetylglucozamin, acetylgalactozamin mostly sulfated and
associated with other types of sugar, uronic acids (iduronate and glicuroni).
The sulphate, carboxylic and hydroxyl groups in their composition confer upon
them an important electronegative charge with an important functional role.
They occupy large spaces and are very hydrophilic; they define hydrated and
porous gels in which the cells are enclavated. Their negative charges attract
clouds of osmotically active Na+ and large quantities of water. The
matrix (140) has great inflation pressure. The porous gels allow the diffusion
of hydro soluble molecules according to their size and electrical charge, such
as hormones, ions, catabolites, etc. There are four groups of special gags by
the composition of sugars, type of connection between them, place and number of
sulfate groups: hialuronan, condroitin, dermatan sulfate, heparan sulfate
cheratan sulfate, heparin. Hialuronan, hialuronic acid locally synthesized
conditions tissue swelling especially in saline medium (62).
Collagen and hialuronan are structural, abundant
components of the connective tissue. These are counterbalanced by the network
of collagen(162). Hialuronan surrounds the neuron leaving a perineuronal space. Glycosaminoglycan
controls bivalent ions (238). So does
heparansulfat (209, 210). Glucuronic acid combined with condroitinsulfate
is also a good absorber of shocks. Condroitinsulfate is very rich in negative
groups and works as an ions exchanger. (2) Proteoglicans (glycosylate
glycoprotein) are constituted of multi sulphurated gags (68), as covalently
connected chains on which many gag chains are fixed like proteins on a protein core , usually a glycoprotein (156).
They are extremely heterogeneous. They have negative electrical charge given by
fragments of sulfated sugars (162). Glycoproteins from the proteoglicans
structure are components of matrix, in interaction with cells. When they have
hialuronan in their structure, they induce the cell membrane. For many types of
cells they act as real receptors (116). They are able to transmit biochemical, hormonal, metabolic, etc signals
which influence the intracellular processes. They are connected to the cytoskeleton. They are the so-called
"mosaic proteins” , spread in the body, some very specialized when they
are included in the constitution of the base membrane. Due to the ability of
gelatin extra cell pericellular matrix,
cavities of various sizes and
with different charges are formed
serving to select the cells and molecules. They play a role in chemical
intercell signaling.
They regulate the activity of other proteins such as protease
inhibitors and proteolytic inhibitors by immobilizing protease in the production place, limiting
the sphere of action by steric blocking
or by creating reserves of proteins, by protection against proteolize. They may increase or
decrease the concentration of proteins by presentation of receptors on the
surface of the cell. They may be associated with the fibrous matrix proteins
(collagen or protein from the network of basal lamina). They interact with
other proteins in ECM as elastin, collagen, fibronectin, laminin, etc.. As
polyanions , they are in interaction with cations and cationic groups. It is
hydrophilic and permits the turgescence of tissues.They are components of
plasmatic membranes. They participate in
cell adhesion and intercellular relationships. The fibronectin binds the cell
with the matrix (5). At the ends there are carboxylic groups. It is also
present in blood plasma, where it participates in the process of blood
clotting.
From the electronomicroscopic point of view there have
been shown physical connections - head to head between filaments of fibronectin
in the ECM and the intracell actin
filaments . The molecule has three fields on the α chains: cellular,
membrane and extracellular having aspartic acid residues of which compete in
the binding of bivalent intracellular cations.
Collagen is the major protein of
ECM, secreted by the cells of connective tissue.
They are fibrous proteins, twisted in the triple helix
rich in proline and glycine. The triple helix (25) originates in the hydrogen
bondings between OH groups of hydroxylizin and hydroxyprolin. In the brain ECM
there is the type IV collagen . The type
IV molecule forms a network included in
the composition of lamina base.
ECM relationship with neurons.
The characteristics of matrix molecules suggest that
they control the function of tissues in which they are located. In the
excitable tissues, proteins have intensely negative charge and interact with
cations.
The ECM interactions are mediated by some matrix
receptors (molecules on the cell surface which bind themselves to the matrix
components). In fact it is not possible to specify the place where the membrane
component ends and where the membrane matrix starts. The last binding between
them is made through a transmembrane protein which binds the matrix to the
citoskeleton. The most important receptors on the cell membrane are very
specialized integrins (laminine, entactine, tensine, etc...), which are binding
to the matrix proteins (collagen) (116).
The integrins binding is weak but their value is given
by their number. Integrins are composed of two subunits and bind themselves to
transmembrane protein matrix. Their intracellular domains are NH2- terminals,
while the matrix ends are COOH-terminals.The extracellular domain matrix
interacts with bivalent cations. On a cell there are several types of
integrines. Their function is very diverse. In order to have the cell to bind
to the matrix, integrines interact with
the cytoskeleton through protein intracell, actine and fibronectine (162).
3.4.
Cerebral matrix.
3.4.1.
General
The organization and the chemical composition show
features that ensure optimal environment for the neuron. It is composed of the
capillary space, the cellular neuron and the glia and by the extracellular
matrix with varying sizes (73). The neurons of the central nervous system are
multipolar, excitable cells. They respond to chemical stimuli, transmiting
excitation to other neurons and to the effectors. At the level of the synapses,
the stimulus is transmitted by means of chemical mediators. Stimuli are encoded
and stored as memory or used by conjugation with other stimuli in mental processes,
etc.. Neurons are constituted of the
pericarion and of extensions (dendrites and axon). The neurons in the
brain are associated in nuclei. Association neurons are entirely situated (including the processes) in the brain
tissue.
3.4.2.
Nevroglia.
Derives, like neurons, from the ectoderm (182). There are six types of glial cells.
(1) Schwann cells, forming the myelin sheath around nerve receptors.
(2) oligodendroglia form the myelin sheath around the axon of nerve centres.
(3) microglia consists of phagocytes, migratory cells in the brain. It removes
cell debris.
(4) the astrocyte regulates the
transition of molecules from blood to the brain.
(5) ependymal cells coat the cerebral ventricle and channel ependima.
(6) satellite cells, which support neurons in the peripheral nervous system.
The astrocyte is a glial cell, great, stellated with many branching extensions
which end as "feet" (187). They represent from 50% in the cerebral
cortex to 90% in many other areas of the total brain volume (73). It is a
polarized cell (128), the extension
being in contact with a cell of mesodermic origin (microvascular
endothelial cell) which it covers (187) and by another extension it is in
contact with the structures of ectodermic origin –neuronal synapse, which it covers . They are ideally positioned
to maintain synaptic activity and energy metabolism of the local environment of
the neuron (126). The astrocyte mediates interaction vessel - neuron, which stabilises them.It has strucural and
molecular characteristics (115). The cytoskeleton of the astrocytes has three
types of proteins (143, 177): Actin microfilaments of 6 nm in diameter,
microtubule of 29 nm diameter microfilaments and intermediate (IF =
Intermediate filaments) with a diameter between 8 -12 nm. Glial IF proteins are
fibrillar, acidic (glial fibrillary acidic proteins = GFAP). GFAP is the most
specific marker for astrocytes in both normal and pathological conditions. The
role of GFAP is given by the large number of anionic groups which is
stabilizing the cytoskeleton, astrocytes maintain shape through interaction
between glial filaments with nuclear and plasma membranes. The ratio of neurons
and noneuronic cells vary by
species,depending on the brain area, on age (128). An astrocyte / neuron ration
= 10 / 1 is specific for most regions of the brain (128). Astrocytes come into
contact with the surface ablumen of the capillary endothelial cells and their
terminal feet capillaries cover the capillary almost entirely (187). The astrocyte
functions are multiple and condition the neuron activity. The astrocyte is
interposed between capillary and neuron and thereby plays an unspecific role of
filter between the capillary and the neuron (Fig.15).
The exchange between these two areas is done through
various mechanisms: diffusion, active transport, electrostatic
interaction, endocitosis, exocytosis,
selective transport imposed by that barrier. Astrocytes play key roles in the
energy distribution of substances in the blood, (128) mediate glucose intake as
a first barrier to nerve cells. Perisynaptic processes incorporate glutamate
during synaptic activity in
somato-sensorial areas. Glutamate is incorporated by astrocites through
a mechanism dependent on Na in the proportion of three cations for a molecule
of glutamate / 128).
Na in the extracellular medium achieves an
electrochemical gradient, so that glutamate is recycled within the glia. The
astrocyte participating in the synapse function
and reincorporation of the neurotransmitter, vesicle fusion ,as well as
its local catabolization. The astrocyte also has a secretory activity. The products secreted
by astrocytes together with the secretion of the endothelial cells are
constituents of the blood-brain barrier. The secretion of the astrocyte
maintains the characteristic of the
blood-brain barrier and of intercell junction . In culture, the astrocyte
secretes laminine, fibronectine, chondroitinsulphate, collagen. The collagen stimulates endothelial cells
maturation. They in turn stimulate the growth of astrocytes. So, with the mixed
secretion of the two cell types the functionality of the blood-brain barrier is
ensured. The network formed by the
" vascular astrocyte processes" also plays a mechanic role by
absorbtion of vascular pulsations, which are no longer transmitted to the
neuron.
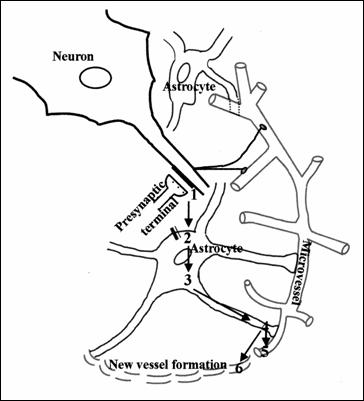
Figure
nr.15. The diagram represents the astrocyte role in angiogenesis and functional
hyperaemia. Glutamate release (1) of neuronal presynaptic that binds to
receptors on astrocyte, activating phospholipase and diglycerol lipase (2) to
release arahidonic acid from astrocytes
(3). The conversion of arahidonic acid to epoxygenase under the influence of
cytochrome C450 2C11 (4). This increases output K + ,which prevents
hyperpolarisation (5) and vascular dilatation. At the same time it increases
Ca2 + intracellularly and stimulates both mitogenesis and angiogenesis. (187)
3.4.3. Cerebrospinal fluid (CSF) (174).
The brain contains 80% water, of which 20% is extracellular water, blood and
CSF, main component of the extracellular fluid. It is secreted by the plexus
ventricular choroid, whose walls are directly permeable for plasma components.
The active transport of plasma solvates has a barrier of the epithelial cells
of the choroid plexus, which regulates the osmotic balance produced by the
passage of water through the existing gradient. Choroid plexuses secrete about
2 / 3 of CSF,the rest being produced by
endothelial capillary (174). The rate of secretion is of 0, 35 ml / min., i.e.
500 ml / day. CSF flows from the lateral ventricles,through
Table 18
Chemical composition ,by comparison to blood - CSF
(78).
CSF blood
Water % 99
93 99 93
protein mg / ml 35 7000
glucose 60 90
Na mEq %o 138 138
K mEq %o 2.8 4.5
Ca 2.1 4.8
pH 7,33 7,41
osmolarity 2950 2950
3.4.4. Lymphatic system
The lymphatic system is one of the ways of
communication between the three areas: Intravascular,interstitial and
endocelular. Lymphatic capillaries begin in the interstitial space, and through
lymphatic vessels carry to the interstitial fluid vessels the result of
capillary filtration, secreted and
enriched with compounds produced at cellular level and metabolytes.It is
absent at brain level.
Chapter 4:
Exchanges Between
Capillaries and Interstices
4.1.
Capillary.
The capillary function is
regulated by chemical and physical factors. The first act in intercellular
signaling ways,by specific transcription factors, proteins, etc.. The capillary wall consists generally of an
endothelial cell layer as a central element programmer (164), situated on the
baseline membrane (174). Endothelial cells form a selective permeability barrier,
secrete some specific compounds involved in metabolic local processes and
regulate contractility of smooth muscle fibers (32). The luminal surface of the
endothelium is covered with proteoglicans, glycoproteins and glycocalyx
composed of fibers. This layer has a thickness of 20 nm and is rich in negative
electric charges (102) with variable density. This layer is covered in turn by
a layer of absorbed plasma proteins forming a 1![]() m thick net, rich in carboxylic groups, sulfate, sugars (acid
N-acetyl neuraminic, mannosil, galactosil, N-acetylglucosamine, sialil residue)
protein glycoconjugat represented by integrine selectine, immunoglobulins. To
endothelial cells are associated proteoglican heparansulphate ,
glycosoaminoglicans. The chemical composition and physical properties are
influenced by local differential adsorption of plasma proteins: albumin,
fibrinogen, orosomucoid. Serum albumin stabilizes endothelium-blood interface,
influences the function of barrier against macromolecules occupying anionic
sites on glycocalyx and endothelium.The perfusion with solution without albumin increases the
permeability of capillaries in muscle and mesenter. Orosomucoide reduces
permeability to macromolecules anionic capillaries in the muscles of rats.
Glycocalyx is a dynamic structure as a barrier to macromolecules. Its structure
and its reactivity vary depending on different vascular territories of change.
The structure of glycocalyx includes the sialic acid and acid glycoproteins
(162). It controls the exchanges cell-capillaries, increases the permeability
for small hydrophilic solvates(33). Therefore the structure and chemical
composition of the wall of the capillary tissue irrigated is important for the
transport in the extravascular space.
m thick net, rich in carboxylic groups, sulfate, sugars (acid
N-acetyl neuraminic, mannosil, galactosil, N-acetylglucosamine, sialil residue)
protein glycoconjugat represented by integrine selectine, immunoglobulins. To
endothelial cells are associated proteoglican heparansulphate ,
glycosoaminoglicans. The chemical composition and physical properties are
influenced by local differential adsorption of plasma proteins: albumin,
fibrinogen, orosomucoid. Serum albumin stabilizes endothelium-blood interface,
influences the function of barrier against macromolecules occupying anionic
sites on glycocalyx and endothelium.The perfusion with solution without albumin increases the
permeability of capillaries in muscle and mesenter. Orosomucoide reduces
permeability to macromolecules anionic capillaries in the muscles of rats.
Glycocalyx is a dynamic structure as a barrier to macromolecules. Its structure
and its reactivity vary depending on different vascular territories of change.
The structure of glycocalyx includes the sialic acid and acid glycoproteins
(162). It controls the exchanges cell-capillaries, increases the permeability
for small hydrophilic solvates(33). Therefore the structure and chemical
composition of the wall of the capillary tissue irrigated is important for the
transport in the extravascular space.
4.1.1. Endothelial capillary.
Endothelial cells united by strong junctions constitute a barrier in the
transport blood - neuron for many compounds including ions (137). Endothelial
cells in the brain are different from those of other territories (21, 22),have
electrical resistance 1.000 ohm between them, while on other territories the
resistance is only 10 ohm / cm (174), are impermeable to macromolecules (50,
249 ), have different mechanisms of transport (22). At this level, the crossing
is by specific processes of endocytosis, mediated by the receptors which bind
the ligand in the beginning and transfer it by internalization in vesicules to
the opposite side, where it is released by exocytosis, and the transfer to
neurons is selected by the blood-brain barrier. The system controls
extravasation cerebral capillary blood components and expansion of
extracellular matrix liquid. There participates in this process also the
polyanionic component of extracellular matrix to maintain the cationic
composition of the neuronal environment (73, 25, 157) and blood component that
controls the activity of Na (231, 233, 236). This protects the neuron against
large variations of the Na gradient. There is therefore a complex control of
transepitelial transport of substance (77). Cell adhesion proteins of brain
capillaries grow by ischemisation which promotes activity in the focal
leucocytes (79). Endothelial cells do not communicate between them.Their matrix
surface is covered by astrocytes with their processes. Another surface of
membrane adheres to the baseline. Through another surface they are in large contact with the blood and
its components. This luminal cell surface is covered, regardless of the
vascular territory, by layers of mobile plasma 1 ![]() m thick (see above). It covers a layer of macromolecules made
up of by proteoglicans, glycoproteins
and by glycocalyx, to which plasma proteins areattached. On this surface there
are to be found Integrines, which provide cell adhesion to the transmembrane
receivers and binds extracellular cell protein. Major vascular Integrines
interact with specific vitronectine and fibronectine, which fixes the cells of
extracellular matrix. Laminine binds the membrane epithelium to the baseline
membrane. The nature of Glycocalyx and the number of anionic sites of the
endothelial cells in the brain differ from that of other vascular beds. A
protein of astrocyte origin induces the
synthesis of proteoglicans in the cell endothelia and increases the selectivity
df blood brain barrier. Due to the negative charge of glycocalyx (98) and
endothelium, anionic molecules penetrate
the brain with more difficulty than
neutral or cationic molecules of equal sizes. On the baseline membrane anionic
sites are less abundant and are part of a mixture of proteoglicans, hydrophilic
amino acids, glycopeptides, sialic acid residues, heparansulphate. In the same
area there are other molecules bound by the membrane: selectine, Integrine,
immunoglobulins, glycolipids, glycoproteins, and proteoglicans, with role in
the inflammatory processes and coagulation. From the reactive composition of
this segment mention should be made of the richness in negative charges of the
carboxylic groups, sulphates, hydroxilic, N- acetil glucosaminic and of
monosacharides: manosil, galactose, fucose. Proteoglicans with long
branched chain are associated to
endothelial cells in a 50 - 90%ration .
Plasma proteins (albumin, fibrinogen, orosomucoid) adsorbed by the capillary
wall, change the properties of chemical and physical-chemical molecular layer
of endothelial cells .The cationisation of serum albumin, immunoglobulin and
antibodies considerably increases the passage of substances in the brain
(Triguero, 1989 , Partridge, 1991). There are no data however to support any role of plasma proteins in the blood-brain
barrier. The arrier is affected by injection in the brain of hypertone solutions in experimental cerebral
hypertension in infections, etc. This process occurs through redistribution or
the loss of anionic sites on the cerebral endothelium. The layer of
extracellular substances, bound to the endothelial cell membrane, covers the
intercellular spaces as a network of plasma proteins adsorbed on the strongly
acid membrane, which binds cations. During the ripening process there takes
place the remodeling on both sides of the endothelium, the development and
maturation of baseline membranes can be a mechanism of differentiation of the
brain microvascular system.
m thick (see above). It covers a layer of macromolecules made
up of by proteoglicans, glycoproteins
and by glycocalyx, to which plasma proteins areattached. On this surface there
are to be found Integrines, which provide cell adhesion to the transmembrane
receivers and binds extracellular cell protein. Major vascular Integrines
interact with specific vitronectine and fibronectine, which fixes the cells of
extracellular matrix. Laminine binds the membrane epithelium to the baseline
membrane. The nature of Glycocalyx and the number of anionic sites of the
endothelial cells in the brain differ from that of other vascular beds. A
protein of astrocyte origin induces the
synthesis of proteoglicans in the cell endothelia and increases the selectivity
df blood brain barrier. Due to the negative charge of glycocalyx (98) and
endothelium, anionic molecules penetrate
the brain with more difficulty than
neutral or cationic molecules of equal sizes. On the baseline membrane anionic
sites are less abundant and are part of a mixture of proteoglicans, hydrophilic
amino acids, glycopeptides, sialic acid residues, heparansulphate. In the same
area there are other molecules bound by the membrane: selectine, Integrine,
immunoglobulins, glycolipids, glycoproteins, and proteoglicans, with role in
the inflammatory processes and coagulation. From the reactive composition of
this segment mention should be made of the richness in negative charges of the
carboxylic groups, sulphates, hydroxilic, N- acetil glucosaminic and of
monosacharides: manosil, galactose, fucose. Proteoglicans with long
branched chain are associated to
endothelial cells in a 50 - 90%ration .
Plasma proteins (albumin, fibrinogen, orosomucoid) adsorbed by the capillary
wall, change the properties of chemical and physical-chemical molecular layer
of endothelial cells .The cationisation of serum albumin, immunoglobulin and
antibodies considerably increases the passage of substances in the brain
(Triguero, 1989 , Partridge, 1991). There are no data however to support any role of plasma proteins in the blood-brain
barrier. The arrier is affected by injection in the brain of hypertone solutions in experimental cerebral
hypertension in infections, etc. This process occurs through redistribution or
the loss of anionic sites on the cerebral endothelium. The layer of
extracellular substances, bound to the endothelial cell membrane, covers the
intercellular spaces as a network of plasma proteins adsorbed on the strongly
acid membrane, which binds cations. During the ripening process there takes
place the remodeling on both sides of the endothelium, the development and
maturation of baseline membranes can be a mechanism of differentiation of the
brain microvascular system.
4.2. The blood-brain barrier (BBB).
BBB is made up of endothelial cells which limit the intraluminal portion of the
brain capillaries, a layer composed of the feet juxtaposition of astrocyte
processes and baseline subepithelial membrane(50, 74). Endothelial cells
present (41, 98) specialized regions by the help of which they form close
contacts (intercellular junction), have high electrical resistance, have no
fenestration, and minor pinocytose activity. In the BBB structure there are
also described both the luminal and abluminal plasma membranes (74).
The endothelium has the role of endothelial barrier to
substances in the blood, the selective membrane for substances in both spaces
,through transcellular mechanisms.The
basement lamina itself forms a barrier between capillary and neuron, is located
between astrocyte and endothelium, acting as a filter . Thickness : 40 -120 nm.
By means of electronic microscopy it is possible to see that it is made up of 2
distinct layers: (5): lamina lucida (lamina rara) adjacent to the plasma
portion of the endothelial cells and of lamina densa, an electrono – dense
layer. A third layer is further described, lamina fibroreticulata, binding the
basement lamina to connective tissue. Basement lamina is a filtrable structure,
polarizes the cell, influences the endothelial cell metabolism, organizes
proteins on the adjacent plasma membrane etc.
It plays a role in synaptic reconstruction, guided nerve regeneration in
time, etc. In the constitution of BBB there is found collagen type IV,
heparansulphate laminine, fibronectin. Between neurons and astrocyte, the
barrier is richer in proteoglicans. Collagen type IV is flexible and forms
networks, stabilized by disulphuric bridges . The membrane also contains agricans secreted by
neurons surrounding the synapsis.
Maintaining the brain function requires
a certain extracellular ionic strength, especially metabolic conditions,
neurotransmitters, growth factors, etc. between certain limits of variation
maintained by the BBB. It accumulates (174) catabolites, ions (specialy K) for
their removal, into the membrane endothelia there is found a large amount of
Na,K-ATPaze. In the astrocyte endings
there is a high density of
channels K (73). At the level of synapsis there are accumulated , during the
nervous pathways activity (sensory, and
of multiple neural circuits ), neurotransmitters which are inactivated by
removal or local metabolisation. From
among these, (128) for example , glutamate that is inactivated by reuptake in
astrocytes perisynapsis with the help of transporters and glial electrochemical
gradients of Na (172).The thus enabled transportation represents a key signal
for coupling neuronal activity with activation of glycolysis in the astrocyte.
The transport of solvates from the blood
into the brain is done either by specific transporters, or in a
transendothelial way , by plexus choroides. BBB provides the necessary amino
acids in the extraneuronal environment and protects neurons by large
fluctuations in the content of Na (174).The change of BBB permeability allows
brain penetration of the same substances that are not transporters, (5) such as
lipophilic molecules, hydrophobic , according to the partition coefficient and
they reach the brain extra cellular space. The barrier allows the passage of
substances also due to the concentration gradient. The size of molecules and
their steric configuration together with the above mentioned factors conditions
the BBB function.
4.2.1.
Adjusting the BBB function.
For the proper functioning of the brain, the energy and plastic substances
requirement makes BBB to respond to cellular signals and to monitor the
transport of substances and ions (74, 28, 99). From the studies on transport
processes it results that strongly polarized substances like glucose, some
amino acids, etc. are crossing with difficulty the lipid membrane of BBB and are transported
by transcellular saturable systems, on
the endothelial cell membranes. The Na+-dependent transport of amino
acids is made by abluminal membrane, and neutral amino acid transport, by
gradient of concentration. In contrast, the transport of neutral aminoacids ,
independent of Na+ is made by both membranes. It is difficult to
quantify transport routes , as their number is very large (87) and they
overlap. BBB is penetrated by cations and cationized antibodies. The function
is dependent of Na.K.ATP and of the saturable transport system operating in
parallel. It is a complex system of incorporation of Na23, when the concentration of Na+ or H+
in the cell increases . It is inhibited by cations Na+, H+,
Li+,
4.2.2. Cerebral territories without BBB.
Circulating hormones influence the central nervous system with access to
neurons in certain areas where the barrier is permeable because of the
endothelial fenestration (187): the posterior hyophysis , medial eminence and
the pineal gland which releases neurohormons from nervous endings. In
capillaries without fenestrations there are many vesicles in cellular cytoplasm
carrying in a transcellular way various substances. In the hypophysis , the
absence of the barrier allows the passing of neurosecretion into the blood. In the subfornix body there is a
chemoreceptor area. The transcellular transport at this level enables balancing
of water and of other homeostatic functions. The regions without BBB are
isolated from the rest of the brain by specialized ependymal cells called
tenocytest , localized along the surface III ventricle , near the median line.
This is the sensory-circumventricular body which includes the vascular organ,
lamina terminalis, subfornix organ, organ subcomisural area postrema, eminence
median, neurohypophysis and plexus choroid. The tonicytes are associated by
tight junctions and prevent the free exchange between circumventricular body
and CSF. Badly bounded area and defined topographically , it also contains some
heterogeneous structures which limit the internal walls of the cerebral
ventricle. Terminalis lamina ending on
the median wall rostral ventricle III contains two circumventricular sensors ,
which control the hydrosalin balance. Ventral of organum vasculosum laminae
terminalis is the recesus supraoptic where the osmoreceptor center is located .
The target place of angiotensin II is
the subfornix organum placed
dorsally and caudally from the above
comisura, enabled by ADH. Therefore it
has a neuroendocrine function and has
highly variable receptors for steroids, angiotensin II, atrial
natriuretic hormone and somatostatin.
4.3.
Kidney territory.
4.3.1.
Renal functional unit.
The structural and functional support of the kidney is the nephron:
vasculo-tubular structure. The vascular part is between the renal artery and
vein including a mirabilis system : the afferent arteriol capillarises first as vascular glomerule Malpighi. The
efferent arteriol capilarises from here a
second time in the peritubular area. It continues as vasa recta to
venula kidney. Nephron begin by Bowman's capsule, invagination of the tube
surrounding vascular glomerulus. It continues through the proximal convoluted
portion, loop of Henle, with a distal
convoluted portion having a segment descending and ascending the following
constituted of a large portion and other thinner, filiform . It continues
through the straight collecting tubules, with the opening to the renal pyramid
. The adjoining vasculo-tubular structures are separated by an interstice of
unequal size. The tubes are made of a
membrane baseline on which there are placed , in the proximal convoluted
portion contort , irregular cuboidal cells, rich in cytoplasm, having protoplasmic extensions on the inner face - brush border. These cells
present invaginations with mitochondria. In the Henle loop , the cells are extremely thin, flattened, with
clear cytoplasm. In the contort distal tubes , the epithelial cells are
cuboidal , smaller , having clear cytoplasm and smooth surface.
The
structure of renal vessels. The glomerular capillaries are in large contact
with the space encapsulation. In the cytoplasm of endothelial cells there are
perforations of 0.1![]() on the internal face and perforations of 100
on the internal face and perforations of 100![]() in the baseline membrane, through which the plasma passes
into the capillary lumen, through filtration. The efferent glomerular vessels
are relatively permeable and also for the molecules of albumin size. The blood
circulation in the area of the thin loop of Henle is upwards, in counter
current with the uriniferous tube. This vessel has a fenestrated epithelium up
to the return of the vasa recta. The interstice between capillary and tubes
consists of some wider and some narrow areas. At the level of the proximal
contort tube , the space is represented by the merger of the membranes of the
tubes and capillaries. Endothelial capillaries present fenestrations of 0.05
in the baseline membrane, through which the plasma passes
into the capillary lumen, through filtration. The efferent glomerular vessels
are relatively permeable and also for the molecules of albumin size. The blood
circulation in the area of the thin loop of Henle is upwards, in counter
current with the uriniferous tube. This vessel has a fenestrated epithelium up
to the return of the vasa recta. The interstice between capillary and tubes
consists of some wider and some narrow areas. At the level of the proximal
contort tube , the space is represented by the merger of the membranes of the
tubes and capillaries. Endothelial capillaries present fenestrations of 0.05![]() , through which the tubular content passes directly into the
blood vessels. Here there take place resorption processes for approx. 74% of
the glomerular filtrate. Otherwise the space is wide. The size of the
interstice is increasing toward the deep kidney regions. Interstice contains
many mucopolisacharides rich in proteins and anionic sites with high basicity ,
which can be modified through changes in loading with water, Na+, or
under hormonal influences. In rats with diabetes insipidus , the basicity of
proteins is increased in the medulla. ADH increases the number of anionic
charges, arresting more and more Na+
, with or without water. The
interstice also contains some serum proteins , brought here by vascular fenestration, mostly albumins . These , together with the basic
proteins , are important in Na+ reabsorption.
, through which the tubular content passes directly into the
blood vessels. Here there take place resorption processes for approx. 74% of
the glomerular filtrate. Otherwise the space is wide. The size of the
interstice is increasing toward the deep kidney regions. Interstice contains
many mucopolisacharides rich in proteins and anionic sites with high basicity ,
which can be modified through changes in loading with water, Na+, or
under hormonal influences. In rats with diabetes insipidus , the basicity of
proteins is increased in the medulla. ADH increases the number of anionic
charges, arresting more and more Na+
, with or without water. The
interstice also contains some serum proteins , brought here by vascular fenestration, mostly albumins . These , together with the basic
proteins , are important in Na+ reabsorption.
4.3.2. The mechanism of urine formation.
The fundamental theory was proposed by Cushing in 1917 and includes three
processes: (1) glomerular filtration of plasma, (2) selective tubular
resorption, (3) tubular secretion. Formation of urine is the result of a
dynamic process of communication between
the three spaces: vascular, interstitial and tubular (222, 137).
Ureogenesis function is done with the participation of hemodynamic factors
(blood pressure, blood output, stroke volume, etc..) membrane factors
(permeability, structure, chemical composition); intratubular speed of the
ultrafiltered and the primary urine, components of the glomerular
ultrafiltered.
|
|
Metabolic factors: general metabolism, acido-basic
status, blood composition, energy dependent transport, simple diffusion
(144), gradients of concentration, electrochemical gradients, molecular
interactions. Physical and chemical factors like colloid-osmotic pressure,
viscosity, protein content, electric charges and molecular polyelectrolyte,
cations. Harmonization of activities of these factors and their adapting to
conditions of the body is regulated nervous and humoral (221, 230) in the
context of the theory of multifactorial urogenesis. |
The endocrine factors have an important regulating
role . Aldosterone acts at the level of the distal contort tube and of
collector tubes. The inactivation of local protein synthesis and of Na.K.ATP is
involved in the Na+ retention. DOCA acts in the same way as aldosterone, but weaker. The
atrial natriuretic hormone (206) increases the glomerular filtration rate,
diuresis and natriuresis , reduces the movement of signals through the point of
departure in the atrial area. It acts at the level of distal portion contort
and of the papillary membrane. Quinine , dopamine, prostaglandins also have
action in the loss of Na, while angiotensin acts directly and indirectly by
stimulating secretion of aldosterone : catecholamines act at proximal and
distal contort level. ADH keeps water by acting at the level of contort distal
portion and collecting tubes on the interstice space. The electrochemical
interaction factors between Na -
proteins act concurrently with oncotic factors, induced by proteins from the
blood and the interstice, which control the tubular and peritubular
reabsorbtion processes through their anionic groups.
4.3.2.1.
Glomerular filtration (primary secretion of urine).
At the level of the glomerule there
takes place the formation of the filtered plasma (1/5-1/3 of the plasma volume) which passes from the
glomerular capillaries into the Bowmann Encapsulation. The ultrafiltrate has
the same composition as plasma, but without proteins having molecular weight
greater than 69,000. Thus a process takes place concentrating proteins in the
vascular postglomerular space, having two consequences: (1) exponential
increase of colloid – osmotic pressure, (2) absolute increase of the number of
anionic protein sites.
Increasing concentrations of protein in the efferent
arteriole has a stabilizing role of blood flow. The glomerular flow decreases
by clamping the aorta (97) and increases under experimental conditions, by
increasing the concentration of protein (albumin) in the blood perfusion (5 -
7%) and intake of hypernatrium. The phenomenon is attributed to the increase of
osmotic pressure over 55 mmHg. Protein increase above 10% leads to a non filtering kidney.
Plasma proteins influence the overall kidney function
depending on its status. The increase of concentration of proteins in perfusion
of the vasodilatated kidney with acetylcholine changes the effect in the loss
on Na of the protein and decreases the
elimination of Na+ and water. The non vasodilated kidney reacts in a
variable mode to the increase of the proteic content of infused protein.
Protein concentration level stabilizes the glomerular renal circulation and
keeps under control the tubular level resorbtion . Patients with analbuminemy
(177, 222), protein malnutrition (59, 141) and those with nefrotic syndrome,
there is a simultaneous decrease of colloid-osmotic pressure and of fractional
Na+ resorption. Increased protein keeps oxygen consumption response
decay of glomerular filtration. Albumin increases from 2.5% to 5% and increases
absolute resorption of Na+ and water. In the absence of albumin ,
the kidney eliminets however, 40 - 46% fraction of filtered water and Na+
, i.e only the minimal regulated
fraction of oncotic pressure . Postglomerular decrease in the proteins produces
volume expansion and kidney proximal reabsorbtion inhibition of Na+.
The mechanisms by which proteins interfere in the regulation of Na+
reabsorbtion can be deciphered taking into account how communication between
the three compartments takes place, a process in which oncotic pressure plays
an important role. The proteins control reabsorption especially of Na+ (with their anionic
charge). Between Na+ and polyanion protein there are interactions
which also definie the fundamental
mechanism of transport of Na+ in the blood (231, 232, 236). In
addition to doubling the concentration of proteins in efferent arteriola and
peritubular capillaries mention should be made of the richness of anionic
groups of interstitial proteins: serum
albumins passed through fenestration of capillaries , mucopolysaccharides rich
in anionic groups.
Their numbers increases under the influence of
increased quantities of Na+ crossing the interstice and from here
into peritubular capillaries , through capillary baseline membrane. The
increase of the number of substances removed, obtained by blood dilution, as
well as the decrease of glomerular flow , diminishes excretion of sodium also
because of the decrease of concentration
of proteins from postglomerular territory. In Henle loop , the Na+
arrested by proteins, creates the
hyperton enviromment necessary for water conservation.
4.3.2.2.
Tubular reabsorption. The role of proteins.
It is a selective process(248), certain substances being preserved,to keep their
blood homeostasis, the so-called threshold substances: glucose, water,
electrolytes, aminoacids, etc.. The tubular function is governed by the
membranes that separate the three compartments and in particular the
composition of proteins, of vascular and
interstitial space. Changes in the intake of any of the substances above are
reflected in the composition of urine, fact that shows that the threshold also depends on the
balance
|
|
An increased intake of glucose (20%) and a null intake of Na+ eliminates urine
with a high volume, but with an extremely low content of Na+ (data
unpublished). Resorption has two phases: the lumen of tubes with its
interstition and the latter with the stroke. The exchange between the tubular
lumen and interstices achieved in a
paracellular and transcellular mode.Na+, although an
extracellular ion, at the level of proximal contorts portion there are located 20mM/Kg of wet tissue, a content
which grows in parallel to the
concentration of the intraluminal filtrate, up to 100m M / Kg , of which 60%
is free and 40% held in the cells. Na+ is carried to the
interstice on a transcellular route in a rate of 20-25%, endergonic. The
paracellular transport is dominant at the Henle loop level, ascending
portion, for Na+. The downward branch is impermeable to Na+
but permeable for water. Paracelular transport is made by diffusion. The
peritubular environment is hyperosmotic, has a rich content in
mucopolysaccharides and basic proteins , serum albumins, rich in anionic
groups that interact with Na+.
In
conclusion, the ureogenetic and homeostasis function of Na+ is
performed by special structures (compartiments) and with the variation of
blood composition and of glomerular filtrate
along nephron and with an important
role of the proteins. These act by their concentration change , by
which they control the colloid - osmotic pressure and also by the richness of
anionic charges , interacting with sodium. |
[Chapter 5 will be featured in the
upcoming January-February 2010 issue of this Journal.]
Professor Marcel Uluitu, M.D. Ph.D. began his
scientific activity in Physiology in 1953 at the
Professor Uluitu has also investigated
cerebral tissue excitability, studying the structure modification of the
protein macromolecules, and the physiological and pathopysiological processes
in which are involved Sodium and Lithium. He implemented an original method for
physical and chemical processes which involve the chemic active sodium, in
normal processes and in the cerebral excitability dysfunctions, in human and in
experimental model (animal). These results of this work gave him the chance to
outline the chapter herein relating to the physiology of substances transport
in the blood. This is based on the physical and chemical interaction between
blood components.
His papers are included in the
collections of the U.S. National Library of Medicine and the U.S. National
Institute of Health. He is a member of the
Dr. Diana Popa (Uluitu) is a
researcher in the Department of Microbiology, Immunology and Molecular Genetics
at the
[ BWW Society Home Page ]
© 2009 The Bibliotheque: World Wide Society