Thalidomide: A Remedy with
Two Faces*
by Prof Dr Dr Randolph Riemschneider, L.B.Fel.
and
Central
Santa Maria, Rio Grande do Sul, Brazil
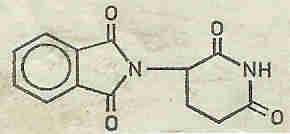
A chemist sees in THALIDOMIDE (plate 1) a substituted glutamic acid derivative, ie a molecule which is structurally related to the amino acids and might possibly be expected to have a positive or negative effect on the intermediary metabolism.
The author details his own
experiences with thalidomide in view of the tragedy, but also its "benefical"
sides (- the editors).
Almost every German who hears of
thalidomide today immediately thinks of the calamitous affair in 1958-61, viz
the birth of many deformed babies throughout the world after the sleeping pill
thalidomide was taken during pregnancy. The other side of thalidomide is less
well known: while it does not heal leprosy, it does contain this
terrible disease. Thalidomide has proved itself as a weapon against malignant
growths and has been used under strict conditions for some years, eg in the
Plate
1: Thalidomide Thalidomide (Contergan) N-Phthalyl-D,L-glutaminsäureimid or 2,6-Diketo-3-phthalimido-piperidin
Growth-inhibition experiments on
ascites in mice in our institute in 1963, using thalidomide prepared in our
laboratory, provided the first indication of its action on malignant cells (3).
In the mid-60s, Prof Dr H Gerhartz noted that it inhibited the formation of new
blood vessels (angiogenesis) (6), as is desirable in the case of growing tumour
tissue. In a number of cases during six years of co-operation with Prof Dr H
Gerhartz of the Klinikum Westend, FU Berlin (5) and another 12 years with Prof
Dr José Mariano da Rocha Filho, director of UFSM university clinic and rector
of UFSM [1974-84] (2), positive results in cancer therapy were obtained
in the clinics named with the preparation of thalidomide manufactured in the
Institute of Biochemistry at the FU Berlin.
Unfortunately, political
developments at the time at the FU Berlin (1968 student revolution) as well as
the change of government in
Investigations on the subject:
"What else can the thalidomide
molecule do?" from 1957
The author took an interest in this
molecule immediately after thalidomide came onto the market as a sedative in
West Germany in October 1957, more precisely in the question: "What is to be
expected from this unphysiological ‘amino acid derivative’?" and especially after
the thalidomide affair, as is evident from the lectures in 1962 and thereafter
(7, 8, 5, 4).
From 1958, we incorporated thalidomide experiments into four of our on-going projects. As the firm CHEMIE-Grünenthal did not make the thalidomide that we had requested (Nov 57) available for insecticide experiments, we synthesized it ourselves, using various methods (9, 16), and developed a useful manufacturing process (11).
The four projects concerned were:
1) AMINO ACIDS, PEPTIDES, here especially: Thalidomide (I) synthesis, I-analogues, I-antipodes – configuration and action of I analogues
2) PEST
CONTROL RESEARCH, here especially: Alternatives to the Chemical Cudgel
3) GROWTH INHIBITION and
PROMOTION, here especially: On tadpoles and through ascites in mice (chemical
analysis of tumours and growth inhibition in malignant cells) – tumour therapy
4) Teratogenicity, here
especially on tadpoles, rabbits – methods for detecting the teratogenicity of
organic compounds
To PROJECT 1: Description of
I-syntheses and of synthesis of I-analogues (12, 8, 17, 11). No differences in
action were observed in the case of I-stereoisomers (8, 11, 9, 8).
To PROJECT 2: Attempts to negatively
influence the metabolism of insects by placement of unphysiological compounds
(in their food) structurally similar to those occurring naturally – here I and
I-analogues in the form of glutamic acid derivatives:
Phenylalanine derivatives like eg N-(x-methylmercaptobenzoyl)-substituted ones and their oxidation products and flourinated phenylglycine peptides Literature: under Project XXI.
Uracil and xanthine derivatives and others based on pyrimidine and purine
Glutamic and aspartic acid derivatives, eg thalidomides
Carrier oligopeptides with D-amino acids in intermediate position, eg L-lysyl-D-tryptophanyl-L-phenylalanine and 61 others
Pyrimidine and dicarboxylic acid derivatives containing F, eg fluor orotic acid, difluoromalic acid diethylester
Nicotinyl ammine acid esters and nucleoside derivatives
151 preparations 1976-85
To PROJECT 3: Deformities were
observed in some cases during experiments to inhibit the growth of Xenopus
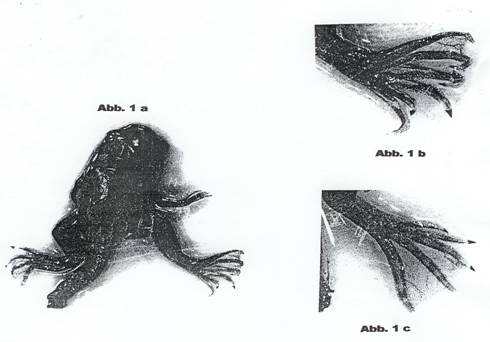
laevis DAUDIN tadpoles (14, 17): Photos: Abb. 1a and 1b. Growth of ascitic
cells in mice inhibited, ie growth inhibition of malignant cells (3). As
mentioned above, according to H Gerhartz [private communication (6)],
thalidomide inhibits the formation of new blood vessels (angiogenesis), as is
desirable in the case of growing tumours: tumour therapy. Initially six years
of experiments in the Klinikum Westend (H Gerhartz) These
photos were made public, among other places, during a lecture given by the
author at the Fiera di Verona in 1983 (cf Ref 17). These photos were sent to
the managers of the firm Chemie-Grünenthal on 01 June 1959, with a request to
support research by a doctoral candidate.
To PROJECT 4: Expanding on the tadpole experiments in 3), and subsequent to Dr Lenz’s lecture (18 Nov 61), gravid rabbits were treated with thalidomide: Observation of the birth of deformed newborn within a few weeks: February till April 1962, published in (15a-d).
The introductory lectures in
It was planned to continue clinical
treatment with thalidomide, but unfortunately this was not possible due to the
change of government and staff, as noted above. Moreover, the author was no
longer able to travel regularly to
From the medical reports and treatment protocols of cancer patients in Brazil (treated 1972-84) on the anti-tumour activity of thalidomide (I) in Refractory Multiple Melanoma: as per handwritten excerpts of the attending physicians, supervised by Prof Dr José Mariano da Rocha Filho of UFSM, Santa Maria, RS, Brazil. Total treatment and observation time from commencement of treatment: up to 52 weeks: "On refractory-myelome-patients (partially previously treated) applied oral I within 12 weaks, starting with 250 mg/day for 3 weeks, then with 200 mg/day for further 3 weeks and later with 150 mg/day until the end. Paraprotein-control experiments: Analysis of Myeloma protein in serum (A), and Bence-Jones protein in urin (sic) (B), Plasma cells number in bone marrow (C). Results of A, B and C: Reduction in 48% of cases."
Bibliography*:
Lectures, reports, manuscripts,
publications by author, J M da Rocha Filho, H Gerhartz inter alia.
(1) R Riemschneider (lecturer), H
Gerhartz, J M da Rocha Filho, K Nolde
Thalidomide – a remedy with two faces
Lecture, given in English at the colloquium
of WIDMER AG, Wädenswil, Switzerland on 15 July 79 on the occasion of the
takeover of the company by ELBIO Co, later BIODELL Co Ltd, Lugano, Switzerland
(scientific manager from 1979: author).
During the lecture, a
detailed report was presented on the results of clinical trials with
thalidomide for treating of tumours, conducted in the Klinikum Westend at the
FU Berlin in 1968-74 and in the university clinic of the UFSM in
Present: Prof Dr J
Mariano da Rocha Filho, rector of UFSM and director of its university clinic;
Prof Dr Helios Bernardi, prorector of UFSM and director of its Institute of
Pharmaceutics; Prof Dr A Lüttringhaus, Organic Chemistry, University of
Freiburg im Breisgau, Germany and director of Widmer, Wädenswil, Switzerland; T
Aikawa, vice-president of Yamakawa & Co, Ltd, Tokyo, Japan.Dr Katajima,
patent lawyer of TEIZO Co, Tokyo, Japan; Dr K Renner, Bayer Werke, Leverkusen,
Germany; Dipl Chem K Nolde [together with Dipl Chem H- J Hein, synthesized the
thaldomide required in the Institute of Biochemistry at FU for years]
(2)
J Mariano da Rocha Filho, R Riemschneider, K Nolde, J Joachimovicz
Report: Clinical results in the treatment of cancer patients (110 cases) with thalidomide 1972-84. 41 cases positive: plasmocytoma or myeloma. (Klinische Ergebnisse in der Behandlung von Krebspatienten (110 Fälle) mit Contergan in den Jahren 1972–1984. 41 Fälle positiv: Plasmozytom bzw. Myelom.)
From the clinic of
the UFSM,
Purest thalidomide
was prepared as previously and delivered by Riemschneider and K Nolde, P
Reichert, R Wasicky,
(3) R Riemschneider, D Takei
Inhibition of growth
of ascites in mice by thalidomide (Hemmung des
Wachstums von Aszites bei Mäusen durch Contergan)
Lab report Jan 64, 11
p, secreted at request of Prof Dr S Takei,
Djunko Takei is the
daughter of the aforementioned professor. It was intended that she continue
this direction of work with other test objects on her return to
(4)
R Riemschneider
"About the thalidomide affair and personal experiences – future research in the field of thalidomide" (Über die Contergan-Affäre und persönliche Erfahrungen – zukünftige Forschungen auf dem Contergan-Gebiet)
Lecture, given to Scientific Colloquium of
Central Institute of Biochemistry and Biophysics at FU Berlin in Oct 74 in
lecture hall of institute building V, Ostpreußendamm 111, Lichterfelde-Süd.
For the reasons given
above, it was – with one exception (5) – impossible for us to research more
exactly into what else the thalidomide molecule was good for and whether
it was possible to develop a sedative
on the basis of a thalidomide analogue, without teratogenic side effects.
We were, however, in collaboration with Prof Dr José Mariano da
Rocha Filho, director of the university clinic of the UFSM,
(5) H Gerhartz, R Riemschneider
Report: Clinical results* when treating cancer patients with
thalidomide in 1968-74 (Klinische Ergebnisse bei der Behandlung von
Krebspatienten mit Contergan in den Jahren 1968 – 1974): 14 positive cases
from ward Vb, oncology in the Klinikum Westend, and Institute of Biochemistry,
both at FU Berlin. Purest
thalidomide prepared in the
Results: Positive results in 14 cases of
patients with breast cancer. Publication was postponed at the time, initially
for reasons of patent law, but also due to the "critical situation" at the Free
University of Berlin. The trials were kept as secret as possible on account of
the "radical" students. It must be emphasized that they were not commissioned
by industry, but were solely a private inintiative by the two aforementioned
befriended professors.
(6) H Gerhartz, director of oncology section
(ward Vb) at Klinikum Westend, FU Berlin
Personal
communication:
Thalidomide, prepared in
(7) R Riemschneider (lecturer)
"About teratogen
action of organic compounds", Bull II: "On modification of thalidomide
molecule (I) to find analogues without teratogenic side effects and test
what else the I-molecule can do" (Über die teratogene Wirkung organischer
Verbindungen", Mitt. II: „Zur Abwandlung des Contergan (Thalidomid)-Moleküls
(I), um Analoge ohne teratogene Nebenwirkungen zu finden und um zu
testen, was das I-Molekül noch kann): Lecture in English at
Universidade Federal de Sao Paulo,
Manuscript July 62, 25 pages, 5 plates and 25
formulas, hectographed 1963 in university press of UFSM,
(8) R Riemschneider (lecturer), E Becker, F R
Pesserl, J Joachimovicz
2 lectures:
I "Modification of thalidomide
molecule and on its isomers and analogues" (Abwandlung des
Contergan-Moleküls und über Contergan-Isomere sowie Analoge)
II "(+)-N-phtalyl-glutamic acid
imide (Ia) and (-)-N-phtalyl-glutamic acid imide (Ib)
(+)-N-(Phthalyl-glutaminsäureimid
(I a) und (-)-N-Phthalyl-glutaminsäureimid (I b)
Testing Ia and Ib for
teratogenicity on rabbits, zebra fish and Xenopus laevis DAUDIN
tadpoles" (Prüfung von I a und I b auf Teratogenität im Kaninchen-Versuch,
an Zebrafischen und an Kaulquappen von Xenopus laevis DAUDIN)
Lectures in Central Chemical Institute of UFSM,
Experiments in the thalidomide project were interrupted and prevented for years by the university reform (so-called democratization of Free University of Berlin) 1969, as noted above, with the exception of (5).
We were interested in
transforming the highly interesting thalidomide molecule, firstly to find
1-analogues without teratogenic side effects and also to see what else the
1-molecule can do: The search for other effects of thalidomide and
its analogues.
According to the
relevant literature, thalidomide has meantime been successfully used against
forms of the infectious disease leprosy Erythema nodosum leposum
[ENL].*
However, the leprous women treated with it were insufficiently informed
about its teratogenic effect – the thalidomide affair was repeated for some of
the lepers!
On use of thalidomide
in combatting cancer, cf reports cited below: (5, 4, 2)
(9) R Riemschneider, K Nolde, P Reichert
Laboratory reports:
Two syntheses of
thalidomide (I) (Zwei Synthesen des
Contergans (I))
a) from
N-phthalyl-D,L-glutamic acid anhydride and urea
a) aus N-Phthalyl-D,L-glutaminsäureanhydrid und Harnstoff in der Schmelze,
b) from
phthalimide-potassium and a-bromine glutarimide
b) aus Phthalimid-Kalium und a-Bromglutarimid,
c) precursors and analogues c) Vorstufen und Analoge
8 reports Dec
57, 14 pages
from the Department of Biochemistry at the Free University of
Experiments to
prepare thalidomide were conducted for several reasons:
1 Thalidomide was to
be included in the practical "Synthesis of biochemically relevant compounds"
under "unphysiological amino acid derivatives" (plate 2)
2 As stated above,
thalidomide was to be included in our research into the action of
unphysiological compounds in the organism of insects, specifically to provide a
less radical alternative to the large quantities of toxicants used in pest
control ("chemical cudgel")*
3 Despite our
inquiry, Chemie-Grünenthal, the manufacturers of thalidomide, had declined to
provide us with thalidomide for research purposes
4 as preliminary
tests for synthesizing optically active thalidomides (11).
(10) R Riemschneider, H Horak
N-phthalyl-D,L-glutamic acid imide (I), viewed as an unphysiological glutamic acid derivative. Some inhibition of Drosophila melanogaster MEIGEN progeny in laboratory tests – test methods, (N-Phthalyl-D,L-glutaminsäureimid (I) – eingestuft als unphysiologisches Glutaminsäure-Derivat. Schwache Hemmung des Nachwuchses von Drosophila melanogaster MEIGEN im Laborversuch – Testmethode)
manuscript Jan 58, 8 pages, cf also Plate 2.
(11)
R Riemschneider, R Wasicky, K Nolde,
2 improved working
instructions for preparing N-phthalyl-glutamic acid imide (thalidomide) and
synthesizing 9 analogues; first attempts to synthesize optically active isomers
(2 verbesserte Arbeitsvorschriften zur Herstellung von
N-Phthalyl-glutaminsäureamid (Contergan) und Synthese von 9 Analogen; erste
Versuche der Synthese opt. akt. Isomerer)
manuscript and laboratory reports from
1960-61 in Portuguese (50 copies, hectographed) following serial tests in the
forerunner of the Central Chemical Institute of the Santa Maria Federal
University (UFSM) in Brazil and the Department of Biochemistry at the Free
University of Berlin (FU); cf also (7, 16).
(12)
R Riemschneider, G Rossi
Bull Vb: "Synthesis
of optically active thalidomide isomers: (+)-N-phthalyl-glutamic acid imide
(Ia) and (-)-N-phthalyl-glutamic acid imide (Ib)" Synthese der optisch
aktiven Contergan-Isomeren: (+)-N-Phthalyl-glutaminsäureimid (I a) und
(-)-N-Phthalyl-glutaminsäureimid (I b)",
manuscript, 1961 15 pages and lectures on 01 Mar 63
lecture 1, given in colloquium of institute
at Free University of Berlin; cf also (11)
(13) R Riemschneider, K
Brockmeyer with J Joachimovicz, F R Pesserl, M M Faria, K Nolde and M M Souza
Teratogenic effect of organic compounds Bull III: Further thalidomide experiments with other breeds of rabbits, with mice of various strains, with golden hamsters, chinchillas, dogs, pigs, (Teratogene Wirkung organischer Verbindungen Mitt. III: Weitere Thalidomid-Versuche mit Kaninchen anderer Rassen, mit Mäusen verschiedener Stämme, mit Goldhamstern, Chincillas, mit Hunden und Schweinen)
Z Naturforschg 18b, 584-585 (1963)
(14) R Riemschneider, N. Schuster, H Horak
Growth- and metamorphosis-inhibiting effect of thalidomide (1) on Xenopus laevis DAUDIN tadpoles: Table 1 (Wachstums- und Metamorphose-hemmende Wirkung von Contergan (I) auf Kaulquappen von Xenopus laevis DAUDIN: Tabelle 1.)
2 laboratory reports
Dec 58 and Jan 59, total 19 p. Deformaties were observed in 2 cases. Photos:
Abb. 1a, 1b, 1c.
Table 1: XENOPUS metamorphosis data
metamorphosis Thalidomide controle
Begin 39
days 47 days
End 55
days 66 days
(15a) R Riemschneider, K Brockmeyer,
H Sommer
About the teratogenic effect of organic compounds Bull 1: Thalidomide, (Über die teratogene Wirkung organischer Verbindungen Mitt. I: Thalidomid)
Z Naturforschg 18b, 167-168 (1963). [Received on 09 Aug 62]. Initially
held back by editors, published only following intervention by Prof Dr
Butenandt; cf also (15b)
(15b) R Riemschneider, C Lange, K Brockmeyer, H Sommer
Experiments to detect the teratogenic effect of thalidomide in rabbit tests (Versuche zum Nachweis der teratogenen Wirkung von Contergan im Kaninchenversuch)
Laboratory reports May 62 – Basis for the tests
described here (15), conducted from Dec 61 to May 62 in the rabbit breeding
facility purchased by the Institute of Biochemistry at FU Berlin from Mr Carl
Lange of Hamburg-Neuhof in collaboration with Drs med K Brockmeyer and H Sommer
as well as Mr Carl Lange (inasfar as his health permitted) and two students
from the University of Hamburg. Results: First indications of the
teratogenic effects of thalidomide on mammals (test animals). The editors of
the journal "Arzneimittelforschung" declined publication in June 62; the jounal
"Zeitschrift für Naturforschung" also only published these results after an
initial delay, following intervention by the Nobel prize winner Prof Dr A
Butenandt: (15a).
(15c) R Riemschneider
Lecture 1: "Proof of the teratogenic effect
of thalidomide in rabbit tests" (Nachweis der teratogenen Wirkung des
Contergans (Thalidomid) im Kaninchenversuch ) with 20 slides of deformaties in foetuses, given in
German to the "Medical Association" (Sociedad de Obstetricia y Ginecologia de
Buenos Aires y Sociedad Argentina para el Estudio de la Esterilidad) in Buenos
Aires, Argentina on 04 Sep 62 and simultaneously translated into Spanish,
extracts published in (15a).
The trip to
(15d) R Riemschneider (lecturer), R Schuster, G Koh
"Study of space models (STUART-BRIEGELB) of N-phthalyl-glutamic acid imide (thalidomide) and analogues from the aspect of teratogenic side effects" (Studium der Raummodelle (STUART-BRIEGLEB) von N-Phthalyl-glutaminsäureimid [Contergan] und Analoger unter dem Gesichtspunkt der teratogenen Nebenwirkungen)
Lecture II given on 11 Sep 62, otherwise as
above (15c).
After lectures I
and II, the following questions were addressed: Which positive aspects can
be wrested from the thalidomide molecule? Transformations of the molecule,
other applications?
(16) R Riemschneider, K Nolde, P
Nowack
Thalidomide from
N-phthalyl-glutamic acid anhydride and NH3 gas (Contergan aus
N-Phthalylglutaminsäureanhydrid und NH3-Gas)
Various laboratory
reports, Feb 58, 17 pages, (unpublished)
(17) R Riemschneider (lecturer), J M
da Rocha Filho, J. Joachimovicz
Teratogenic effect of
organic compounds
Manuscript Jan 63 and 4 lectures: In
Sao Paulo (Oct 61 [1182],
Some of the
illustrations were published in conjunction with a lecture given at the "Fiera
dell’Agricultura" in
These investigations
are the continuation of the experiments from 1958-59 (14, 18) in which the
inhibition of metamorphis in Xenopus laevis DAUDIN tadpoles had already
been observed. Further investigations in this direction together with Dr W
Trost and Dipl Biol R Roetz.
[During the discussion about teratogenic
effects which took place subsequent to the said lecture in Verona, the author
said: "Nella mia relazione ho accennato ai girini (Kaulquappen) come animali
molto sensibili, per verificare effetti teratogeni di un prodotto. Tanti anni
fa, abbiamo controllato sperimentalmente il prodotto contergan, riscontrando
capacità teratogena. Nella foto Abb 1a che vediamo, si puo costatere una rana
con una zampa alterata della presenza di 6 dita anziché le normali 5. La foto
Abb 1b è un ingrandimento della zampa alterata. Questa ed altre malformazioni
sono comparse sui girini trattati con contergan in breve tempo."]
(18) R Riemschneider (lecturer), N Schuster
"Inhibition of growth
in Xenopus laevis DAUDIN tadpoles and delay in their metamorphosis by 9
- 12 days under the influence of thalidomide" (Hemmung des Wachstums der
Kaulquappen von Xenopus laevis DAUDIN
und Verzögerung ihrer Metamorphose um 9 – 12 Tage unter Contergan-Einfluß)
lecture II[1] of Oct 61 and manuscript May
59, 10 pages: Teratogenic effects were observed in some cases on frogs
photographed at the end of metamorphosis: analogous to photos: Abb. 1a-c. As we
had no experience in the fields of embryopathy and teratogenicity, these
observations were not accorded the significance they deserved. But despite
their negative attitude, we sent this information with photos (Abb.1) to
Chemie-Grünenthal, the manufacturers of thalidomide, in Jun 59 (14) requesting
funds for a doctoral candidate. Unfortunately, no answer was forthcoming at the
time. Continuation of experiments: (17).
* Based on a lecture by author in English on 15
Jul 79 to colloquium of WIDMER AG, Wädenswil,
* original titles: bold
* The author actually considered conducting
further experiments with test animals before using in the clinic.
However, Prof Gerhartz pointed out that this required special approval and
would lose a lot of time. It would be easier to begin directly with clinical
trials, particularly as Contergan [Softenon in
* Licensed in
* PROJECTS VI-XI of publication by author quoted
in Plate 2
[1] Lecture I, also in
[ BWW Society Home Page ]
© 2004 The BWW Society/The Institute for the Advancement of Positive Global Solutions