Stereochemistry Cis-Trans
Asymmetrical Compounds
Axis-Ring
Molecular Aggregates[1]
by Dr. Randolph Riemschneider, L.B.Fel.
Professor Emeritus of Biochemistry and Chemistry
Here the author presents two projects from the field of stereochemistry
named in the title. Both were realized in the 50s to early 60s, though only the
results of the cis-trans asymmetry were published in detail at the time.
Reports on synthesized axis-ring molecular aggregates were solely in lectures
and a patent application. -- The editors.
We speak of cis-trans asymmetry
in the case of a so-called asymmetric C-atom if two of its four different
substitutents are geometrically isomeric, but symmetric: Fig. 1. In
1955 - 64, it proved possible to realize several such cases where geometrical
and optical isomerism coincided: Pl 1.
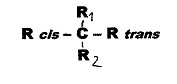

Fig.1:
cis-trans-asymmetric compounds
The synthesis of several axis-ring molecular aggregates, ie "compounds" without a stable bond between "axis" and "ring" (Fig 2) is described, first carried out in 1953 - 64: Pl 2. Applications are discussed, equally the reasons for deferring publication of the early results.
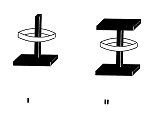
Fig.2:
diagrammatic representation of axis-ring
molecular aggregates:
type I and II ("semi-dumbbell","dumbbell")[2]
Plate 1: Synthesized cis-trans asymmetric compounds: I, IV, IX, XII
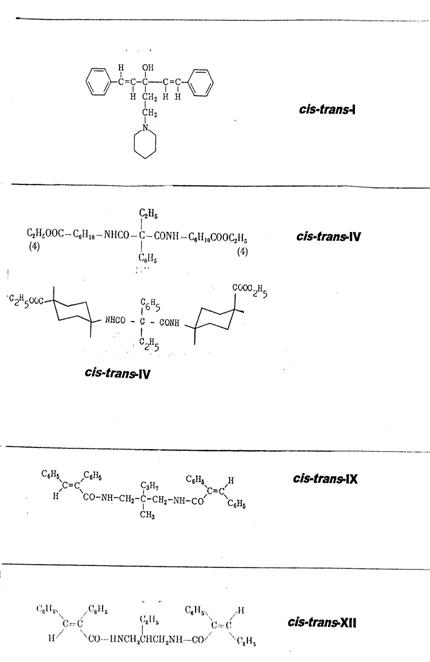
Plate 2: Axis-ring molecular aggregates XIX, XII, XXIV and V
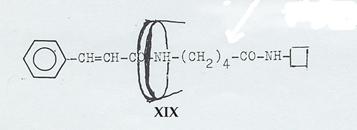
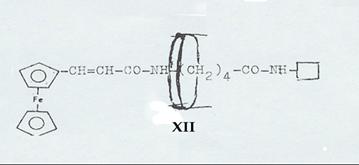
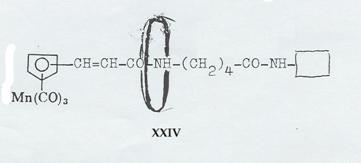
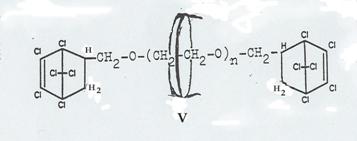
XIX, XII, XXIV realized (Pl 7 - 9), but
not V (Pl 10)
I cis-trans asymmetric
compounds
The following pathways were taken to synthesize the compounds characterized in Fig. 1:
1) cis-trans double bonds were produced by partial hydrogenation in a system of type:
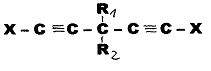
Example 1 (Pl 3)
2) “Aufhänger” - “Anhänger” principle (hanger-pendant principle):
“Aufhänger” 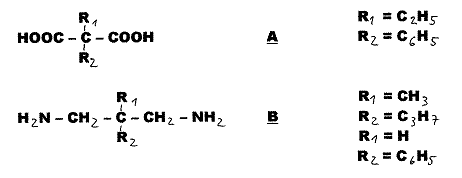
“Aufhänger” A and B were converted according to the so-called hanger-pendant principle. In the case of A in stages via monoacidchloride with cis- and trans-1,4-diamino-cyclohexane-carboxylic acid and in the case of B in stages with cis-trans-cinnamic acid chlorides: Examples 2 - 4, Pl 4 - 6.
In all cases, care had to be taken from the outset that the synthesized compounds were projected in such a way that they permitted splitting into dextro and laevo components, so as to detect the expected optical activity.
The four synthesized cis-trans asymmetrical compounds are listed in Pl 1, details in Pl 3 - 6 with keys; cf. also III Appendix.
Example 1:
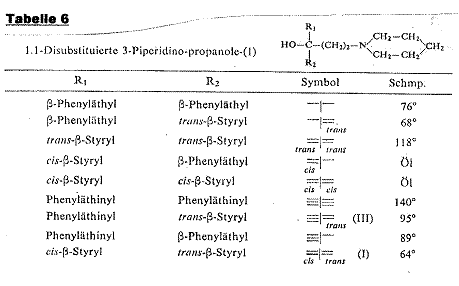
1-(cis-β-styryl)-1-(trans-β-styryl)-3-piperidino-propan-1-ol [cis-trans-I] (2a, b): Pl 3
The reasons for selecting Example 1 are:
1) The geometrically isomeric ligands are in maximum proximity to the centre of asymmetry (requirement of “Entfernungssatz”)
2) Optical activity by reason of steric hinderance is impossible, as observations of space-filling models (STUART and STUART-BRIEGLEB) show
3) Optically active phenyl and vinyl carbinols with considerable amounts of rotation, as described in the literature, led us to expect “sufficient” rotations for styryl carbinols
Plate 3:
Synthesizing 1-(cis-β-styryl)-1-(trans-β-styryl)-3-piperidino-propan-1-ol
[cis-trans-I]
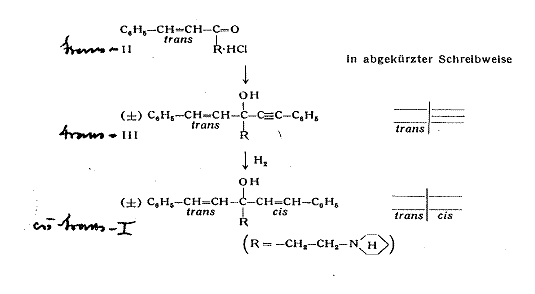
Key to Pl 3:
trans-benzalacetone was converted in a MANNICH reaction with formaldehyde and piperidine hydrochloride to 1-phenyl-5-piperidino-trans-pent-1-ene-3-on (II) to synthesize I. Phenylethinyl-magnesium bromide reacts with the ketone II to 1-phenyl-ethinyl-1-trans-β-styryl-3-piperidino-propan-1-ol (III) from m p 96oC and is split into the optical antipodes with (-)-dibenzoyl tartaric acid by fractional crystallization of the salts. Finally, the triple bond of both antipodes is selectively hydrogenated with a LINDLAR catalyst to the cis double bond; cf Appendix
Example 2:
Ethyl phenyl-malonic acid-cis-(4-carboxy-cyclohexyl)-trans-(4-carboxy-cyclohexyl)-amide [cis-trans-IV] (3, 4): Pl 4
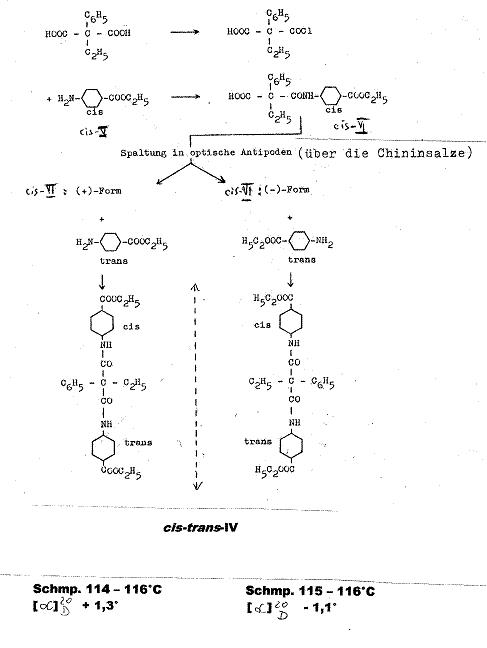
Key to Pl 4:
The monochloride obtained by boiling ethyl-phenyl-malonic acid and SOCl2 in diethyl ether for 12 hrs was converted with cis-4-amino-cyclohexan-carboxylic acid-diethyl ether (cis-V) in the presence of triethylamine in CHCl3 at 0oC to racemic ethylphenyl-malonicacid-mono-(cis-4-carbethoxy-cyclohexylamide) (cis-VI). The antipodes from racemic cis-VI were seperated using their quinine salts in ethyl acetate, from which (-)-cis-VI and (+)-cis-VI were obtained.
When acted on by p-nitrophenol and N,N-dicyclohexyl-carbodiimide,
the latter compounds yielded the corresponding cis-VII-cyclohexlamide-p-nitrophenyl-esters,
which were converted with trans-4-amino-cyclohexane-carboxylic acid
diethyl ester (trans-V) into
(-)-ethyl-phenyl-malonic acid-cis-(4-carboxy-cyclohexyl)-trans-(4-carboxy-cyclohexyl)-amide;
(-)-cis-trans-IV from mp 115 - 116oC and [M]![]() - 5.6°; [α]
- 5.6°; [α]![]() - 1,1o (α: -0.11o; c = 0.595g
in 10 ml CHCl3; 2 dm) and
into (+)-ethyl-phenyl-malonicacid-cis-(4-carboxy-cyclohexyl)-trans-(4-carboxy-cyclohexyl)-amide
(+)-cis-trans-IV from mp 114 - 116oC [M]
- 1,1o (α: -0.11o; c = 0.595g
in 10 ml CHCl3; 2 dm) and
into (+)-ethyl-phenyl-malonicacid-cis-(4-carboxy-cyclohexyl)-trans-(4-carboxy-cyclohexyl)-amide
(+)-cis-trans-IV from mp 114 - 116oC [M]![]() + 6.7o; [α]
+ 6.7o; [α]![]() + 1.3o (α = 0.13o; c = 0.500 g in
10 ml CHCl3; 2 dm).
+ 1.3o (α = 0.13o; c = 0.500 g in
10 ml CHCl3; 2 dm).
cf.: III. 2 Experimental Part to example 2 and explanation in plate 12
Symmetric, optically inactive cis-cis-IV was formed analogously from (+)-cis-VI and (-)-cis VI with cis-4-amino-cyclohexane-carboxylic acid diethyl ester.
The in comparison with the racemic form significantly higher mp of the optical antipodes of cis-trans-diamide, whose mixed melting point was over 20o lower, was noteworthy, and further underpins our findings. Racemic cis-trans-IV mp 79 - 81oC. It was obtained from racemic trans-monoamide (trans-VI) and cis-V and from racemic cis-VI and trans-V. Mixed mp of both racemic cis-trans-IV preparations: 78 - 80oC. Further details and confirmational formulas for IV and precursors in Appendix.
Example 3:
1-(cis-α-phenyl-cinnamoylamino-3-(trans-α-phenyl-cinnamoylamino)-2-methyl-2-n-propyl-propane [cis-trans-IX] (5, 6): Pl 5 a, b
Plate 5a: Synthesizing 1-(cis-α-phenyl-cinnamoylamino-3-(trans-α-phenyl-cinnamoylamino)-2-methyl-2-n-propyl-propane (cis-trans-IX)
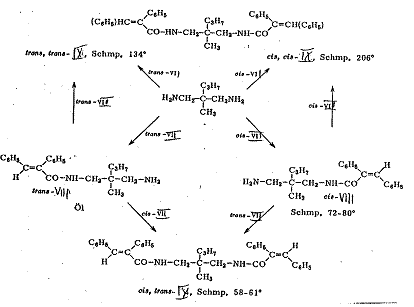
Plate 5b: Seperating racemate 3-(cis-α-phenyl-cinnamoyl-amino)-2-methyl-2-n-propyl-proylamine (cis-VIII) from Pl 5a
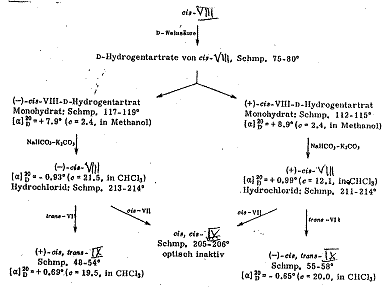
Key to Pl 5a, b
The reactions of cis- and trans-phenyl-cinnamic acid-chloride (cis-VII and trans-VII) with 3-methyl-2-n-propyl-1,3-diamino-propane into the 3-(trans-α-phenyl-cinnamoylamino)-2-methyl-2-n-propylpropyl-amine-Isomers: (trans-VIII) and 3-(cis-α-phenyl-cinnamoylamino)-2-methyl-2-n-propyl-propylamine (cis-VIII) as well as their reactions with cis- and trans-VIII into cis-trans-IX are presented in Pl 5a.
The reaction of cis-VIII with tartaric acid for resolution into the antipodes (-)-cis-VIII and (+)-cis-VIII as well as their reactions with cis-trans-IX, cis-cis-IX and trans-trans-IX are formulated in Pl 5b.
Classification as cis- or trans- was done by comparing the UV spectra of trans-trans-IX, cis-trans-IX and cis-cis-IX (maxima: 280 mμ; 284 mμ) with those of cis and trans stilbenes (maxima: 280 mμ; 295 mμ), but bearing in mind that the trans-phenyl-cinnamic acid (configuration: trans position of carboxyl group to β-phenyl group) corresponds to the cis stilbene and the cis acid to the trans stilbene.
In our case too, the wavelength of the peak of the compound with trans stilbene structure is longer than that of the compound with a cis stilbene structure, whilst that of the cis-trans compound is between both, ie classification by spectrascopy squares with the course of the synthesis.
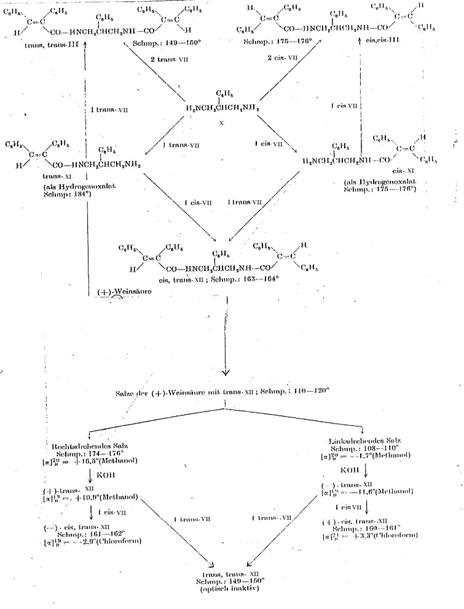
Key to Pl 6:
1,3-diamino-2-phenyl-propane (X) was converted with cis- and trans-α-phenyl-cinnamic acid chloride (cis- and trans-VII) analogously to the process described for 1,3-diamino-propane (140). The noncrystallizing monoacyl compounds (cis- and trans-XI) were characterized as hydrogenoxalates.
Work was continued on the hydrogenoxalate of 2-phenyl-3-(trans-α-phenyl- cinnamoylamino)-propylamine (trans-XI-hydrogenoxalate). The base precipitated from it by KOH was converted with (+)-tartaric acid. It was possible to seperate the diastereomeric salts by means of their differing solubilities in hot isopropanol and further purify them by subsequent repeated crystallization from methyl and ethanyl alcohol. The optically active monoacyl derivatives (+)-trans-XI and (-)-trans-XI liberated from the salts by KOH were brought to reaction with cis-VII, forming two cis-trans-substituted optically active compounds, namely (+)-cis-trans-XII and (-)-cis-trans-XII. On the other hand, when the two optically active trans-XI isomers were converted with trans-VII, we obtained - as was to be expected - optically inactive trans-trans-XII preparations that were identical with each other and with authentic 1,3-bis-(trans-α-phenylcinnamoyl-amino)-2-phenylpropane. This result also proves the identity of the two optically active trans-XI isomers.
The individual reactions, melting points and specific rotations of the optically active compounds are given in this plate (Pl 6).
Example 4:
1-(cis-α-phenyl-cinnamoylamino)-3-(trans-α-phenyl-cinnamoylamino)-2-phenyl-propane [cis-trans-XII] (7, 8): Pl 6
Plate 6:
Resolution of racemates from 2-phenyl-3-(trans-α-phenyl-cinnamoylamino)-propylamine (trans-XI) and synthesis of 1-(cis-α-phenyl-cinnamoylamino)-3-(trans-α-phenyl-cinnamoylamino)-2-phenyl-propane (cis-trans-XII)
II Axis-ring molecular agggregates
The author explored the question of axis-ring molecular aggregates outlined in Fig.2 in a series of lectures, round-table talks and discussions from 1943 on [overview in references (9, 10)] and pointed out ways of synthesizing such "compounds". In personal talks, Profs Richard Kuhn and Lüttringhaus encouraged him to realize projects like the one stated, i.e. (9g,1)
II.1 Recovery and analytical chemistry
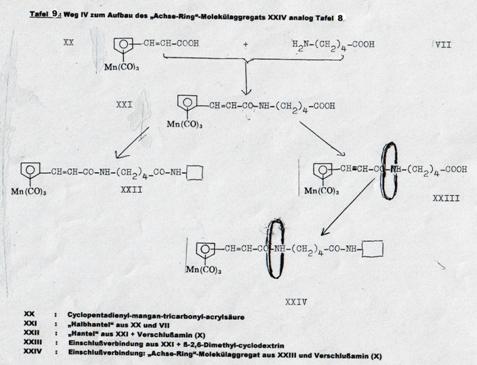
Four pathsways were taken to synthesize such molecular aggregates in 1953 - 58, as shown in Pl 7 - 10. It proved possible to realize three of the four desired end products (Pl 2), viz the aggregates XIX, XII and XXIV; it was not possible to obtain aggregate V (Pl 10): Pathway I.
The type-II molecular aggregates synthesized (Fig 2) were:
XIX: Dipeptide from trans-cinnamic acid, δ-amino-valeric acid and naphthylamine, ringed with cyclodextrin (Pl 7): Pathway II
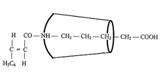
XII: Dipeptide from ferrocenyl acrylic acid, δ-amino-valeric acid and closing amine, ringed with cyclodextrin (Pl 8): Pathway III
XXIV: Dipeptide from cyclopentadienyl-manganese-tricarbonyl-acrylic acid, δ-amino-valeric acid und closing amine, ringed with cyclodextrin (Pl 9): Pathway IV
The elucidation of the iodine colour reaction and inclusion compounds by F Cramer et al [Ber dtsch chem Ges 83 296 (1950), 86 1576, 1582 (1953)] in the 50s suggested considering cylodextrins as ring systems – in fact, as our own experiments showed - above all the β-2,6- and γ-2,6-dimethyl-cyclodextrins (12, 14, 11).
The pathway first taken (Pl 10), using diallyl glycolether as the axis and closing it by reaction with hexachlorocyclopentadiene, forming hexachlorobicycloheptene systems (15), did lead to stringing a cyclodextrin ring together to the "semi-dumbbell" IV (Pl 10) but not to the end product desired: "dumbbell" V, as the reaction temperature required for adding the second hexachlorocyclopentadiene molecule caused decomplexation.
The pathways II–IV presented in Pl 7 - 9 were positive, where peptide closed on one side was ringed and then closed with a differently structured component to become a "cyclodextrin-dipeptide dumbbell".
Plate 7: Synthesizing the axis-ring molecular aggregate XIX: Pathway II
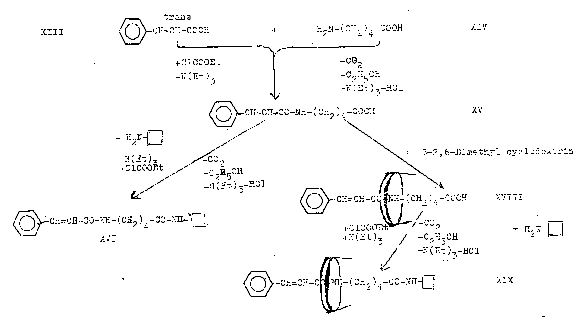
III
= trans-cinnamic acid
XIV
= σ-amino-valeric acid
XV = peptide from XIII and XIV
XVI = "dumbbell" from XV and closing amine XVII
XVII = closing amine
XVIII = inclusion compound from "semi-dumbbell" XV and β-2,6-dimethyl-cyclodextrin
XIX = axis-ring molecular aggregate ("dumbbell") from XVIII and closing amine XVII
β-2,6-dimethyl-cyclodextrin represented in XVIII and XIX
![]() by
by
Fig 3:
Diagrammatic representation of "semi-dumbbell" molecular aggregate XVIII with β-2,6-dimethyl-cyclodextrin (Pl 7)
Cyclodextrin is represented differently in Pl 7, which predates this diagram (fig 3)
Plate 8:
Synthesizing the “axis-ring” molecular aggregate XII: Pathway III
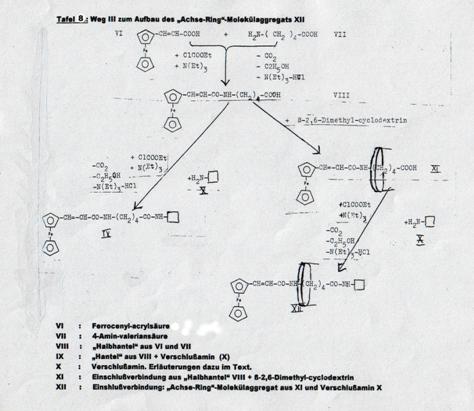
Plate 9:
Synthesizing the “axis-ring” molecular aggregate XXIV: Pathway IV
Key to Pl 7 - 9:
Reaction of "semi-dumbbell" compound XV with β-2,6-dimethyl-cyclodextrin to XVIII [type I compound] (Fig 2): A suspension of 0.01 mol XV (Pl 7) in 75 ml tetrahydrofuran (THF) is treated with agitation with 0.01 mol β-2,6-dimethyl-cyclo-dextrin. Product XVIII obtained the next day after destilling off the solvent in vacuo is dried at 40oC.
C70H115O38N (XV : cyclodextrin = 1:1)
C calc 59.7% found 60.0%
H calc 7.29% found 7.1%
N calc 0.9% found 0.8%
TLC: Rt value of XVIII at 0.15; of cyclodextrin: 0.63. - Merck TLC-plates
IR: 1712 (C=O), 1650, 1528 amide bands; 1609: (C=C)
3280 - 3550: OH bands of β-2,6-dimethyl-cyclodextrin
according to labreports; synthesis of XV described in (13)
Synthesizing the"axis-ring" molecular aggregate XIX (Pl 7) [working with 0.001 mol quantities (1:1:1:1)]: ClCOOC2H5 is laced into the solution of XVIII from the above experiment and triethylamine in 100 ml THF at -20oC and caused to react after several hours of agitation with β-naphthylamine (or other bulky amines).
After 48 hrs of agitation, processed to XIX by destilling off the solvent in vacuo and treating the residue with diethylether.
C80H123O37N2 (XVIII : cyclodextrin = 1:1)
C calc 56.3% found 56.0%
H calc 7.2% found 7.4%
N calc 1.6% found 1.5%
TLC: Rt value of XIX 0.0; of cylodextrin: 0.62
IR: Amide bands 1655, 1532
Elimination of β-dimethyl-cyclodextrin from compounds XVIII and XIX by the action of amyloglucosidase:
XVIII and XIX are treated with amyloglucosidase at 40oC in an incubator and processed after 48 hrs by adding water, destilling off, washing and drying in vacuo.
The IR spectra of the reaction products obtained from XVIII and XIX indicates the lack of the cyclodextrin component.
Plate 10: Synthesizing the “axis-ring” molecular aggregate V: Pathway I
Key to Pl 10:
As noted above, the pathway taken here had to be broken off in the last stage, ie the synthesis of V from IV, as the reaction temperature necessary for "closing" the "semi-dumbbell" cyclodextrin inclusion compound caused "decomplexation" of compound IV.
The bis-alkyl ethylene glycol synthesized with Koetzsch, and its mono- and di-adducts with hexachlorocyclopentadiene, are described at (15). The complexations of the monoadducts with β-2,6-dimethyl-cyclodextrin and its γ-analogues was carried out as described in the key to Pl 7.
Comparison of properties of "dumbbell" molecule XIX with ring and "dumbbell" XVI without ring (Pl 7):
Solubility in CHCl3: XIX soluble, XVI slightly
HPLC retention times: XVI greater than XIX
IR spectra (OH valence): XIX positive, XVI negative
Unexpectedly for us, XIX was remarkably stable, even in solutions like ethyl ester acetate, CHCl3 and methanol/H2O (HPLC) and only dissociates on warming in components ring/axis.
All the compounds and aggregates dealt with in Pl 7 - 9 were synthesized
anew by H-J Hein and M Z Azhar in 1973 - 78 and further secreted by ETHYL Corp
of
XIX's HPLC curve has no peak for the ring-free compound XVI, further proof of the former's stability. A mixture of XVI and β-2,6-dimethyl-cyclodextrin has no XIX-peak: no complex formation of XVI with cyclodextrin during the experiment.
Analysis of the NMR spectrum indicated the successful enzymatic elimination of β-2,6-dimethyl-cyclodextrin from XVIII and XIX. Experiments with XII and XXIV led to corresponding results.
1H-NMR spectroscopic investigations were conducted jointly with Prof Morino into all axis-ring molecular aggregates to test the temperature-dependent interactions of ring and axis, here example from Pl 7:
There is a shift in the NMR signals for the inward-pointing C3-H-protons of the β-2,6-dimethyl-cyclodextrin ring for the "semi-dumbbell" molecule with "ring" [XVIII, Pl 7] compared to the corresponding "semi-dumbbell" molecule without cyclodextrin [XV]: the C3-H proton signal of the free ring compound XV spread after transition into the axis-ring complex and shifted downfield.
Above all the results [250 MHz, CDCl3] obtained with a low-temperature NMR spectrum of the cyclodextrin-containing "semi-dumbbell" molecule XVIII were interesting: the mobilty of the "threaded" cyclodextrin is reduced at lower temperatures - the C3-H-proton signal downfielding by approx 0.05 ppm at -40oC, when comparing the NMR spectra of the cyclodextrin-containing "semi-dumb-bell" molecule at +20 and -40oC.
Differential Scanning Calorimetry:
DSC - an advanced method of differential thermal analysis - was particularly well-suited for characterizing the thermal behaviour of type I and II "axis-ring" molecular aggregates (Fig. 2), Dipl-Chem H-J Hein and M Fereira taking great pains with it in our institute - including for teaching biochemistry (22).
Cyclodextrin-containing "axis-ring" molecular aggregates were compared with a mixture of cyclodextrin and a ring-free component and with a ring-free component alone.
There is a peak above the
melting signal of XV for XVIII, the "semi-dumbbell" with
cylodextrin (Pl 7), no signal corresponding to XV for it, and only a
signal for XV in the 1:1 mix of cyclodextrin and XV. There are no signals for
cyclodextrin in the temperature limits up to 220oC (22). Im Falle der „Halbhantel“ mit
Cyclodextrin XVIII (Tafel 7) tritt ein Peak auf, der über dem
Schmelzsignal liegt, das XV zeigt; das XV entsprechende Signal tritt im Falle
XVIII nicht auf, im Gemisch 1:1 aus Cyclodextrin und XV taucht nur das
Signal für XV auf. Cyclodextrin zeigt in den Temperaturgrenzen bis 220°C keine
Signale (22).
We compared the signal of the cyclodextrin-containing "dumbbell" molecule XIX (Pl 7) with that of the cyclodextrin-free component XVI. There was a corresponding signal for XIX as well as for XVI, but no longer for XIX's second melting, indicating its decomplexation (22).
As noted above, the "axis-ring" molecular aggregates XII and XXIV (Pl 8, 9) were measured by DSC and compared with their 1:1 mixtures as well as with the ring-free components. Here, too, the signals found proved the presence of genuine axis-ring compounds, ie the curves correspond to the observations and interpretations made above. A not genuine axis-ring molecular aggregate is represented in Fig 4.
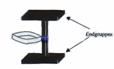
Fig 4:
Model of a theoretically possible, undesired, not genuine type III axis-ring molecular aggregate.
 III
III
Key to Fig 4:
Axis-ring aggregate II and its precursor I are explained in Fig 2. We could not initially preclude the possibility of III being formed instead of II, or indeed of a mix of both. All investigations available to us to date tend to indicate that XIX, XII and XXIV are genuine axis-ring aggregates: (19-24)
II.2 Applications and publishing problems
The water solubility of the cyclodextrin-free components was improved considerably by the take-up of cyclodextrin rings in the type I and II "semi-dumbbell" and "dumbbell" molecules (fig 2), so making it possible to give the relatively non-polar ones a marked hydrophilic character, which is significant for polymer chemistry - appropriately designed axis-ring molecular aggregates could be polymerized in aqueous solution, without using an organic solvent. It ought to be possible to make "non-polar" polymers water-soluble by lengthening the "axes" and so increasing the opportunity of taking up more than one cyclodextrin ring.
Being able to couple "active ingedients" to cyclodextrins and later release them enzymically also presents interesting applications in medicine and pharmacy.
All these application claims were already submitted in Dipl Ing O Matter's June 56 patent application (11).
The many years of secreting the results (at the request of Ethyl
Corp of
After the long years of forced break, the author resumed investigations
in
Mr Azhar had saved most of the preparative and analytical results
obtained after resuming work were on disks. They were all[3]
were destroyed by his sister, who had come to
Prof Morino, meanwhile 80 years old, was no great help in the matter, especially as he had lost his sense of hearing and was no longer able to write articles: cf cit (22, 23).
It should not remain unmentioned that some publications have meantime appeared in this field under the title "rotaxane", led by the article by H SCHILL, G ZÖLLENKOPF, Nachr Chem Techn 1967 186; later H OGINO, J Amer chem Soc 103 1304 (1981); D S LAWRENCE, J S MAMKA, J Amer Chem Soc 112 2440 (1990); R S WYLLE, D H McCARTMEY, J Amer Chem Soc 114 3136 (1992); R ISNIN, A E KAIFER J Amer Chem Soc 113 8188 (1991).
Prof Dr Lüttringhaus complained in a phone call in Jan 70 that the two first-named authors above had not cited RIEMSCHNEIDER's investigations into axis-ring molecular aggregates (known from lectures) although one of them knew about them, according to one co-worker of Lüttringhaus. Prof Lüttringhaus attributed it to the wretched situation the new university law had brought German science to. Over 700 people working at the Free University of Berlin (FUB) were nominated professors under this law at the end of the 60s. The talk was of August or discount professors who - in the view of Prof Lüttringhaus - "will presumably still burden the state in 2000", as he put it in his call to the author.
Prof Lüttringhaus was aware of our investigations into axis-ring aggregates from 1948 on (9g). The author always kept him informed.
III APPENDIX
III.1 Experimental to Example 1 [Pl 3 (2a, b)]:
Hydrogenating (-)-1-[trans-β-styryl]-1-phenylethinyl-3-peperidino-propan-1-ol to
(-)-1(cis-β-styryl)-1-(trans-β-styryl)-3-peperidino-propan-1-ol
3.455 g (-)-1-[trans-β-styryl]-1-phenylethinyl-3-peperidino-propan-1-ol
are hydrogenated in 50 ml
reagent-grade methanol with 1.5 g LINDLAR catalyst until the calculated
quantity of H2 (242 ml at 22oC/760 torr) is taken up. The
filtered-off methanol solution is evaporated in a dark room in vacuo. The
remaining oil only partially crystallizes, the oily portion is seperated off with a little petroleum
ether. It alone rotates the level of the polarized light. The (mixed) melting
point of the crystalline portion is identical with the body formed on hydrogenating the inactive carbinol. The
oily fraction cis=I=trans showed [α]![]() = -36oC (c=7; petroleum ether).
= -36oC (c=7; petroleum ether).
An oil is formed in like manner from carefully purified (+)-1-[trans-β-styryl]-1-phenylethinyl-3-peperidino-propan-1-ol:
![]()
Combining the two antipodes: If the two optically active oils obtained above are mixed 1:1, the mixture crystallizes after a while. The crystals formed (m p 64oC) are (per mixed sample) identical with the cis=I=trans obtained by hydrogenation from inactive 1-[trans-β-styryl]-1-phenylethinyl-3-peperidino-propan-1-ol.
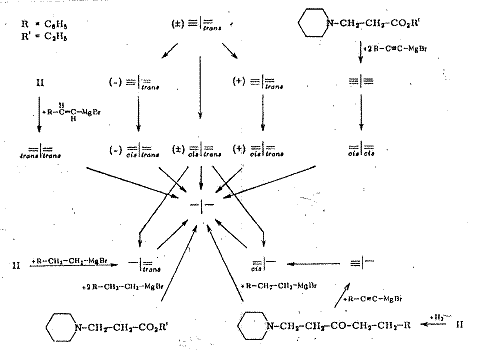
The following stereoisomers were synthesized

to check the configuration and constitution of compounds summarized in Tab 6 and Pl 11.
All the compounds described could be returned to the common saturated parent substance from b p 76oC by hydrogenation:
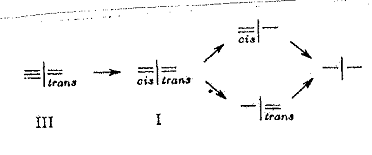
Plate 11:
Hydroderivatives, dehydroderivatives and isomers from cis=I=trans-I (table 6)
UV and IR spectra as well as the change in optical rotation on taking up H2 upon hydrogentation from (-)-III to (-)-I, and via an intermediate stage on to --/-- were considered further proof:
Steric assignation of the two double bonds: the three isomers of I took the same quantity of H2 up on hydrogenation and resulted in the same –I– ; the m ps differ considerably; the UV spectra behaved as expected: fig 4 and 5
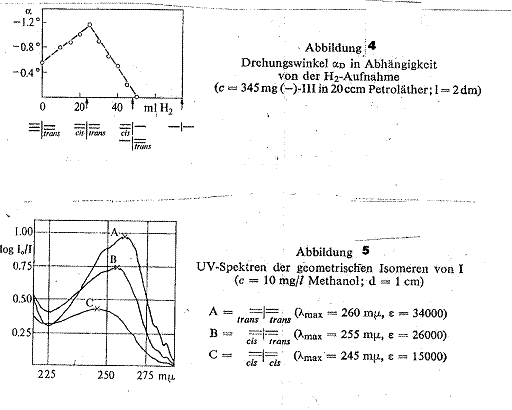
To conclude, a report on the total hydrogenation of active ≡ I= (III) focusing on the rotation:
If the rotation is plotted against the H2 take-up (Tab 7), a curve is obtained which rises until a take-up of 1 mol H2 and falls back to zero on taking up a further mol H2. We had expected that the rotation would only fall back to zero on taking up three mol H2. We believe an explanation for this early disappearance of the rotation may be that cis =I – and –I = trans with opposite rotations form and exactly compensate each other. The curve speaks for the formation of cis=I=trans (2b).

Angle of rotation dependence on volume of
H2 taken up: 345 mg probes of
![]()
![]() in 20 ccm methanal were hydrogenated
in 500 mg Lindlar cataylst respectively till taking up 10, 15, 20, 25,
30, 35, 40, 45 and 50 ccm H2, resulting in a maximum rotation of
in 20 ccm methanal were hydrogenated
in 500 mg Lindlar cataylst respectively till taking up 10, 15, 20, 25,
30, 35, 40, 45 and 50 ccm H2, resulting in a maximum rotation of ![]() α = rotation of 345 mg
substance in 20 ccm petroleum ether in 2-dm tube.
α = rotation of 345 mg
substance in 20 ccm petroleum ether in 2-dm tube.
Table 7: H2 take-up on total
hydrogenation with Pd(OH)2/BaSO4
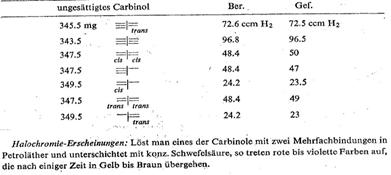
Halochromic effect: If one of the carbinols with two multiple bonds is dissolved in petroleum ether and sulphuric acid is added to form a lower layer, red to violet colours appear that change to yellow to brown after a while.
III.2 Experimental to Example 2 [Pl 4 (3, 4)]
One peculiarity in connection with the synthesis of IV was that it
was difficult to prepare the monoacid
chloride of ethyl-phenyl-malonic acid from m p 58oC. The last
publication on monoacid chloride from dicarboxylic acid, namely the
mono-chloride from malonic acid, was published by
Ethyl-phenyl-malonic acid-monochloride:
Pour 90 ml absolute ether over 15 g ethyl-phenyl-malonic acid from m p 161 - 162oC, add 10.5 g SOCl2 (molar ratio 1:1.25) and heat for 12 hrs on water bath till simmering. Inspissate the reaction solution in water-jet vac on water bath at 20oC bath temp and add approx 150 ml low-boiling petroleum ether to the oily residue - after a while 3 g starting product seperate out. Filter solvent, distill off at 20oC in vac to remove any SOCl2, take up residue in petroleum ether, treat with active carbon and evaporate the solution to low bulk, store for a while until at 0oC ethyl-phenyl-malonic acid-monochloride crystallizes out as colourless cubes: mp 58oC (ie decomposition point), yield 10 g, ie 77% rel to converted dicarboxylic acid.
Classifying the isomeric 4-amino-cyclohexane-carboxylic acids and their acyl derivatives in the cis- or trans series is certain thanks to the investigations of J Houben and A Pfau, Ber dtsch chem Ges 49 2294 (1916), L Orthner and R Hein (Biochem Z 262 461 (1933), G Wendt (Chem Ber 75 425 (1942) and E Ferber and H Brückner (Chem Ber 76 1019 (1943).
As the acid saponification in mild conditions of the ethyl ester prepared from the amino acids and their hydrochlorides returned to the respective starting product only, the steric classification can be considered valid for the ethyl esters too.
Spectroscopic investigation of the constitution of the amides prepared confirmed their constitution. Comparison of the IR photos of cis-cis-IV, trans-trans-IV and cis-trans-IV as well as cis-VI and trans-VI shows that the compounds in the cis- and trans-series have the same configuration.
The differing steric alignment of the COOC2H5 group in the cis- and trans-forms is evident from the homogenous shifts in the CO valence vibration on cis-trans transition: cis-cis-IV and cis-VI have a strong band at 1186 cm-1, to be found in the corresponding trans-compounds trans-trans-IV and trans-VI with decreased intensity of 1255 - 1260 cm-1; as expected, cis-trans-IV shows both absorptions of the ester groups. In contradistinction to these shifts in absorption, no changes in the maxima of the amide groups are noted (3d).
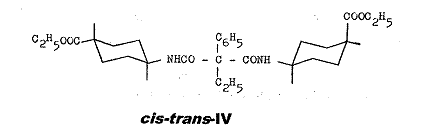
The conformation of cis-trans-IV is explained in Pl 12:
The isomerism of cis-trans-IV can be attributed exclusively to the e or a position of the COOC2H5 group; cf. key to Pl 4.
Plate 12:
Explanation of conformation of cis-trans-IV: ethyl-phenyl-malonic acid-cis-(4-carboxy-cyclohexyl)-trans-[4-carboxy-cyclohexyl]-amides
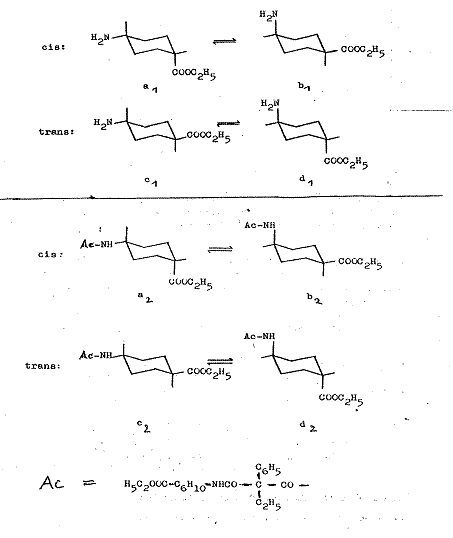
Key to Pl 12:
The conformations a1 and b1 with equatorial amino groups and axial carbethoxy group, or vice versa, is the consequence for the cis form of VI, whilst both functional groups can be present in equatorial (c1) or axial (d1) position in the trans form; PROJ. XIX and IX in (1).
Generally, type a and b or c and d conformations are respectively in equilibrium as "body" and "counterbody", but only a few kcal/mol are needed for reciprocal reaction, so it is impossible to isolate them with conventional chemical methods. These general regularities do not, however, preclude the following electrostatic interactions or particular steric conditions tipping the equilibrium substantially, even towards an unfavoured conformation in the presence of polar groups.
If one now considers the bis-(cis- and trans-4-carbethoxycyclo-hexamide) and the cis-trans-bis-(4-carbehoxycyclohexyl-amide) of ethyl-phenyl-malonic acid, the pendant (as the N-acyl derivative of 4-aminocyclo-hexane-carboxylic acid in cis- and in trans form) has a mainly a2 and c2 conformation in which the acylated NH2 group is the largest substituent in the sterically favoured equatorial position.
IV Bibliography:
The list is very detailed to acknowledge the services of numerous colleagues and institutions.
(1) R Riemschneider
Re-reading 66 years of chemistry: 26 projects and over 1400 references (in prep)
Ref here to PROJ II.3: cis-trans Asymmetry (1a, b, c, e) and PROJ II.7: Axis-ring molecular aggregates (1b, c, d, f). Cf also other PROJs
(1a) R Riemschneider
"Degree
of asymmetric and optical rotation - "slight" differences in similar substituents: H-D, H-T, cis-trans,
syn-anti, endo-exo"
Ms 1941, 25 p, lecture given in chem colloquium, Dept of Org Chem, Univ of Leipzig on 29 July 41 (coll chaired by Prof Dr B Helferich; present: Profs Drs H Bredereck, C Weygand, F Hein, Kautsky; A Papadopolous [Greece], Enrique Lafosse Benedetti [Peru] inter alia)
(1b) R Riemschneider
"Molecular asymmetry and formulating new stereochemical problems"
Lecture given in frame of colloquium at (German) Army
Explosives Research Est in
Ms Feb 43, 22 p
The lecture explained that only two cases of isomerism can be differentiated in stereochemistry:
geometrical and optical. We were particularly interested in the cases where optical and geometrical isomerism coincide, like the so-called cis-trans asymmetry (Fig 1).
The synthesis of type I and II axis-ring molecular aggragates was also dealt with in detail in the 1943 lecture (Fig 2).
The ms and the 29 July 41 lecture (1a) were later
expanded to the author's public inaugural lecture ("demonstration
lesson") at Friedrich Willhelm Univ of
(1c) R Riemschneider
"Molecular
asymmetry"
Lecture given, concluding habilitation proceedings at
Friedrich Wilhelm University of Berlin (meantime renamed Humboldt Univ of
The lecture defined cis-trans compounds as having a central atom with four different substituents, of which two are geometrically isomeric (but symmetrically structured). The energetic disparity of geometrically isomeric substituents could be expected to be sufficient to prove optical activity in this type of compound.
In the discussion after the inaugural lecture questions were raised about realizing such cases of cis-trans isomerism, namely by Prof A Dinghas (Maths) and Prof F Neunhöfer (Org Chem).
It was possible to point out several concrete paths that would lead to "cis-trans-asymmetric" compounds using some prepared slides.
(1d) R Riemschneider
"Stereochemical
consideration on heptites (model experiments) and on axis-ring molecular aggregates"
Lecture given in grand auditorium, Inst of Zool, Free Univ of Berlin on 05 May 50 (panel chair: Profs Drs G Schenck and A Dinghas); cf PROJ II in (1): Tab 9, 10 and ref 222 – 225.
(1e) R Riemschneider (lecturer), O Göhring, H
Arnold, E Hausmann, W Stuck
"Pathways
to synthesizing cis-trans asymmetric compounds:
"hanger-pendant"
principle"
Colloquium lecture II
to leading scientists from Montecatini works in
Ms Jan 51, 29 p
Examples of cis-trans isomeric pendants and description of synthesizing hangers taken into consideration for a while, such as dihydroxy-pivalic acid and α,α-p-xylylene-diglycine (characterized as α,α-p-xylylene-diglycine-dimethyl ester-dihydrochloride from m p 220oC).
Cf lectures and exposé from 1943 and 1948 (1 b - d).
(1f) R Riemschneider (lecturer), G Orlick, T
Siebenmark
"Pathways
to synthesizing axis-ring molecular aggregates"
Lecture given to directors and leading scientists,
Montecatini works, Via Turati,
Ms Apr 52, 19 p + 6 pl
Present: Prof Dr G Natta, Tech College of Milan [later Nobel laureate for low-pressure polyolefins, together with Prof Dr K Ziegler]; Prof Dr A Coppadoro, director of Milan journal "La Chimica e Industria"; two directors of Montecatini works [two of the "four greats"]; Prof Dr D Marotta, director of Instituto Superiore di Sanità (Higher Inst of Hygiene), Rome and Prof Dr H Alessandrini.
(2a) R Riemschneider, K Brendel
Bull IX: cis-trans asymmetry of 1,1-bis-[β-styryl]-3-piperidino-propane-1-ol
Angew Chem 73 655 - 656 (1961)
(2b) R Riemschneider, K Brendel, J Takei
On cis-trans asymmetry
Bull XII:
1,1-bis-[β-styryl]-3-piperidino-propane-1-ol
Liebigs
Ann Chem 665 43 - 54 (1963)
(3a) R Riemschneider, D Kirstein
On cis-trans asymmetry
Bull X: ethyl-pehyl-malonic acid-α-β-bis-(4-carboxy-cyclohexyl-amides)
Z Naturforschg 17b
522 - 524 (1962)
(3b) R Riemschneider, D Kirstein
On cis-trans asymmetry
Bull Xa: Hydrogenating aminobenzoic acid to cis-4-amino-cyclohexane-car-boxylic
acid (α form) and trans-4-amino-cyclohexane-carboxylic acid (β form);
[α-hydrochloride, m p 217oC; β-hydrochloride, m p 279oC]
Ms Jan 61, 8 p (unpublished)
(3c) R Riemschneider, D Kirstein
On cis-trans
asymmetry
Bull Xb: ethyl-phenyl-malonic acid-monochloride
Mh Chem 94 419 - 421 (1963); cf III.2
(3d) J T Shimozawa, R Riemschneider, D
Kirstein
Stereoisomer
asymmetry
Bull Xc: IR spectroscopic examination of the cis-trans isomers from Bull X, Xa and Xb
Ms 1962, 8 p; cf. (1) PROJ. II. 3
(3e) R Riemschneider, D Kirstein
On cis-trans asymmetry
Bull Xd: ethyl-phenyl-malonic
acid-α-β-bis-[4-carboxy-cyclohexyl-amides
Ms 1962, 25 p - with detailed description of experiments + 10 plates and tables; short report cf. (3a) and here III 2: Experimental to Ex. 2, Pl. 4.
(4) R Riemschneider (lecturer), H Kampfer, A
Rook, K Brendel, D Kirstein
Bull XIX: "About several cases of cis-trans asymmetry"
Lecture given in colloquium of Central Inst of Chem, Fed Univ of Santa Maria (UFSM), Santa Maria, Rio Grande do Sul, Brazil on 03 Aug 73 (in Portuguese), based on Bull on cis-trans asymmetry (1, 2b, 3 a - e)
(5) R Riemschneider, H Kampfer
Bull VIII: optical activity of a cis-trans asymmetric compound
Z Naturforschg 16b
704 (1961)
(6) R Riemschneider, H Kampfer
On cis-trans
asymmetry
Bull XI: optical activity of cis-trans-1,3-bis-[α-phenyl-cinnamoyl-amino]-2-methyl-2-n-propyl-propane
Liebigs Ann Chem 665
35 - 42 (1963)
(7) R Riemschneider, A Rook
On cis-trans
asymmetry
Bull V: 1-cis-3-trans-bis-[α-phenylcinnamoylamino]-2-phenyl-propane
Naturwiss 48 500 (1961)
(8a) R Riemschneider, A Rook
Bull VI: mono- and diacylation of 1,3-diamino-propane with stereoisomeric acid chlorides
Mh Chem 92 1197 - 1200 (1961)
(8b) R Riemschneider, A Rook
Bull VII: detecting the optical activity of 1-cis-3-trans-bis-[α-phenylcinnamoyl-amino]-2-phenyl-propane
Mh Chem 92 1227- 1234 (1961)
(9) R Riemschneider
Various lectures on axis-ring molecular aggregates and other stereochemical problems: (9 a - g)
(9a) lecture in
(9b) lecture
in
(9c) lecture in
(9d) lecture in
(9e) three-part lecture in
- "Sterically hindered ethers"
- "Stable reaction partners of high-substituted chair configurations
- "Axis-ring aggregates"
Ms 1954, 18 p + 8 plates (slides)
hectographed after translation into Portuguese [50 copies]; here Pl 7 - 9; present: Prof Dr José Mariano da Rocha Filho, later rector of Univ of Santa Maria, Brazil (USM > UFSM); Prof Dr R Wasicky, panel chair; Prof Dr J Joachimovicz, later USM > UFSM, as well as several professors of organic chemistry from the Univ of São Paulo, Brazil
(9f) lecture in
Axis-ring aggregates - no strong bond between axis and ring [Diag 2, here Pl 2 and 7 - 10], given in English at invitation of rector Mariano da Rocha Filho in rector's office, Univ of Santa Maria (USM) (10 - 20).
Confirmation of and thanks for lecture by letter from Ministerio da Educacão Cultura (Min of Cult Educ), Univ of Santa Maria, Reitoria (rectorate), signed by Prof José Mariano da Rocha Filho.
Translated Copy:
Prof Dr R Riemschneider
Bolivarallee 8
Dear Professor Riemschneider
We hereby thank you for your lecture "axis-ring aggregates" in the field of stereochemistry, given on 15 July 61. The clear and critical presentation of your results met a very positive auditorium, likewise your introduction into stereochemistry itself.
In view of the subject, we had invited competent
guests from the
Yours faithfully
(signed) Jose Mariano da Rocha Filho
Rector,
(9g) R. Riemschneider (lecturer)
Exposé about the following subjects
- “Stereochemical asymmetry”
- “Sterically hindered ethers”
- “Stable partners of high
substituted chair configurations”
- “Axis-ring molecular aggregates”
Oct/Nov 1948, 21 p. (unpublished),
round-table talks,
(10) R Riemschneider (lecturer)
Three-part lecture:
- "Chain-ring aggregates of Type I and II" (Fig 2)
- "Experiments
to synthesize chain-ring aggregates based on Tab 7 - 10"
- "How to realize and analyze the prepared aggregates?"
Lecture given in Aug 1959 at round-table talks to scientists of ETHYL Corp,
(11) R Riemschneider (inventor), O Matter
(applicant)
Draft patent: "Synthesis and application of 'axis-ring' molecular aggregates”, applied for at Swiss patent office by Dipl Ing O Matter in June 56, Prof Dr Randolph Reiemschneider named as sole inventor
Patent assigned to ETHYL Corp of
Main claim: Synthesis of "axis-ring molecular aggregates from cyclodextrin(s) as 'ring' components that do not enter into a strong chemical bond with the 'axis'. Chains that are closed on one or both side by bulky residues and contain hydrocarbon or gylcolether chains as well as peptide bonds serve as 'axes' for 'threading' cyclodextrin(s) and cyclodextrin derivatives on. Several types of 'axis' are described in the subclaims, eg the compounds in Plates 7 - 10.
Applications claimed:
- increasing water-solubiliy of non-polar compounds for both low- and high-molecular compounds
- enabling polymerization in aqueous solutions without organic solvents
- connecting active medical ingredients to cyclodextrin rings of "axis-ring aggregates" and later enzymatic liberation
In Aug 56, the text of the patent specification (without claims) was sent to the following befriended Austrian, German and Italian professors: Nobel laureate Prof Dr R Kuhn, Nobel laureate Prof Dr A Butenandt, Prof Dr A Lüttringhaus, Prof Dr Staudinger, Prof Dr W Lautsch, Prof Dr T Wieland, Prof Dr F Weygand, Prof Dr F Wessely, Prof Dr D Marotta, Nobel laureate Prof Dr G Natta as well as to Prof Dr R Wasicky, São Paulo, Brazil.
(12) R Riemschneider, E Schölzel
Revising bibliographical references on the behaviour of cyclodextrins (β, γ) and derivatives as "host" molecules for various "guest" molecules: preliminary work for synthesizing "axis-ring" molecular aggregates,
lab log 1954, 15 p.
Simultaneously working out experiments with inclusion compounds (iodine of starch, iodine-cyclodextrin reaction inter alia) for "basic biochemical practical" at the Free University of Berlin from 1953 on, above all the sections "carbohydrates" and "inclusion compounds"; hectographed scripts distributed to participants till 1968, then printed as book (four editions by 1987).
(13) R Riemschneider, M Crivelli
N-(trans-cinnamoyl)-5-amino-valeric acid (XV in Pl 7)
Lab report 1954, 4 p (lab log 1954)
0.1 mol trans-cinnamic acid, 0.1 mol triethylamine in 150 ml tetrahydrofuran are laced with 0.1 mol chloroformic acid ethyl ester with agitation at -20oC and 0.1 mol 5-amino-valeric acid added after 3 hrs, reprocess after 48 hrs: yellow crystals from m p 121oC
C14H17NO3 C calc. 64,5
% found 64,1
%
H 7,60
% 7,5
%
N 0,63 % 0,59
%
cis-cinnamic acid reacts analogously
(14)
R Riemschneider, A Küchenmeister, E
Schölzel, H Horak
Preparing β-2,6-dimethyl-cyclodextrin (I) and
experiments: "threading" I onto hydrocarbon and glycolether chains
and NH-CO groups containing chains: "semi-dumbbell" models
Lab report 1955, 17 p (secreted)
(15) R Riemschneider, H-J Koetzsch, H D Otto
Preliminary tests
into closing "axes" by NH-CO bonds and by forming adducts with hexachlorocyclopentadiene
3 lab reports 1955, 14 p, partially published in
Mh Chem 91 41 - 47 (1960), 90 787 - 791 (1959); cf Oct 58 lab reports [ref (755) in (1)]
(16)
R Riemschneider, H-J Hein, A Kühnl, H
Helm, H G Kassahn, E B Grabitz, G Koh
Continuing
experiments to close "axes" in frame of work on "axis-ring"
molecular aggregates; closures with
hexachlorocyclopentadiene, sandwich compounds with COOH group (Fe-, Mn based),
aniline derivatives such as 4-phenylaniline, naphthylamine and substitution products
Lab reports, mostly on other matters, 1957, 24 p, partially included in examples for patent application (11).
(17) R
Riemschneider, A Kühnl, G Koh
Further tests
on β- and γ-cyclodextrin and several derivatives for suitability
as "ring" component(s) for synthesizing "axis-ring" molecular
aggregates
Lab report 1958, 11 p (secreted for ETHYL Corp,
β-2,6-dimethyl-cyclodextrin and its γ-analogues proved particularly well-suited for being "threaded" on long-chain "semi-dumbbell molecules, closed one side", ie for forming type I inclusion compounds (Fig. 2) (12, 14, 16).
(18) R Riemschneider, W Stuck, O Matter, H
Klopfer, A Kühnl, G Koh
Pathways taken to synthesize axis-ring molecular aggregates in stages: Pl 7 - 10
Ms 1957, 28 p [secreted as (17)]
(19) R Riemschneider
Experimental
proofs for generation of genuine axis-ring molecular aggragates
- by ultimate analysis (constant molar ratio of "axis" to "ring")
- by TLC
- by IR spectroscopy
- by slitting the cyclodextrin ring open enzymatically
Ms 1958, 12 p [secreted as (17)]
(20) R Riemschneider, H-J Hein, M Azhar, G Koh, P
Wunderlich, M M Faria
Reworking the synthesis of axis-ring molecular aggregates XIX, XII and XXIV realized in 1954 - 58 (Pl 7 - 9; cf Pl 2)
Lab reports 1970 - 80, 52 p: preparations for the following investigations
(21) R Riemschneider, M Azhar, H-J Hein, P
Wunderlich
Experimental proofs of the constitution of axis-ring “compounds” in (20)
Lab reports 1970 - 80
(22) H-J Hein, R Riemschneider, P Wunderlich, M
Azhar
Investigating the behaviour of axis-ring molecular aggregates (Pl 7 - 9) by differential thermal analysis (DTA), to wit by advanced differential scanning calorimetry (DSC)*
*MARTI et al.
"Angew Chem Thermodynamik und Thermoanalytik", (Experiments suppl
37),
Ms 1981, 12 p (on disc)
(23)
R Riemschneider, Y Morino, M Azhar
Further proofs of the constitution of type I and II axis-ring molecular aggregates ("semi-dumbbell" and "dumbbell" models), to wit for the compounds IV, XVIII, XIX, XI, XII, XXIII, XXIV in Pl 7 - 9. NMH analyses, thermoanalyses (DSC), continuation of 1953 - 58 investigations from (12) on, discs (Azhar).
(24) Y Morino, R Riemschneider, M Azhar
NMR analyses - continuation of (23)
Lab reports on NMR measurements and evaluations by Prof Dr Y Morino 1980 - 86, saved to discs by M Azhar; original lab reports remained with Azhar (and were destroyed by his sister after his death).
Notes:
[1] Axis-ring molecular
aggregates are known as rotaxanes in post-1967 literature (refs in Sect II.2).
As our investigations predate this, we have decided to retain "axis-ring
molecular aggregates" - the term chosen by us - for this review.
[2] There is no strong
chemical bond between "ring system" and "axis". I is the precursor
of II, whose terminal groups prevent the "enclosed ring" from
slipping off. Slide from
[ BWW Society Home Page ]
© 2006 The BWW Society/The Institute for the Advancement of Positive Global Solutions