Medicine:
Medical Research:
Protein
Synthesis in the Colonic Epithelial Cells of Aging Mice
as Revealed by Electron
Microscopic Radioautography
by Dr. Tetsuji
Nagata
Department
of Anatomy and Cell Biology,
Shinshu
University School of Medicine, Matsumoto, Japan
ABSTRACT
For the purpose of studying the aging
changes of macromolecular synthesis in the colonic cells of experimental animals,
we studied 10 groups of aging mice during aging from fetal day 19 to postnatal
month 24. They were injected with 3H-leucine, a precursor for protein
synthesis, sacrificed and the colonic tissues were taken out, fixed and
processed for light and electron microscopic radioautography. On many
radioautograms the localization of silver grains demonstrating protein synthesis
in colonic epithelial cells in respective aging groups were analyzed
qualitatively. The number of mitochondria per cell, the number of labeled
mitochondria with silver grains in each cell in respective aging groups were
analyzed qualitatively and quantitatively in relation to the aging of animals.
The results revealed that the protein
synthesis as expressed by the number of silver grains in cell nuclei, cell
organelles, changed with the aging of animals. It was demonstrated that the
number of mitochondria increased from embryonic day 19 to postnatal newborn day
1, 3, 7, 14, adult month 1 and 2, reaching the maximum, then decreased to
senile year 1 to 2. On the other hand, the number of labeled mitochondria
showing protein synthesis at various ages increased from embryonic day 19 to
postnatal newborn day 1, 3, 7, 14, adult month 1 and 2, reaching the maximum
and decreased slightly to senile month 6 to senile year 1 and 2. To the
contrary, the labeling index increased from embryonic day 19 to postnatal day
1, 2 and 3, then decreased to day 14 and month 1, and increased again to month
6, 12 and month 24, indicating the aging changes. These results demonstrated
that intramitochondrial protein synthesis in the colonic epithelial cells
increased, then decreased and again increased due to aging of individual
animals depending upon the cellular activities at respective aging stages.
Based upon our findings, available literature on macromolecular synthesis in
mitochondria of various cells are reviewed.
INTRODUCTION
The colon is a part of the large intestines
in animals and men, among the digestive tubes between the small intestines and
rectum. The mucous membrane of the colon does not form any folds like the small
intestines or the last portion of the large intestine, i.e. the rectum. The colonic
mucous membrane consists of the simple columnar epithelium forming intestinal
crypts but not such villi as in the small intestines. Therefore, the colonic
mucous membrane has a smooth surface, that is lined by simple columnar
epithelium with a thin striated border. The intestinal crypts, or the glands of
Lieberkuehn are straight tubules, which attain a greater length in the colon
than in the small intestines. The colonic epithelial cells differ from the small
intestines in their greater abundance of goblet cells. The epithelial cells
proliferate at the bottom of the crypts where undifferentiated proliferating
cells exists.
We have studied the macromolecular
synthesis of the colonic epithelial cells in the aging mice in 10 groups of
litter mates of both sexes, each 3, from embryonic day 19 to postnatal day 1,
3, 7, 14, month 1, 2, 6, 12 (year 1) and 24 (year 2), by means of light and
electron microscopic radioautography. We first studied the DNA synthesis in the
colon and caecum of aging mice from embryonic day 19 to postnatal month 12 by using
3H-thymidine [1, 2, 3]. Light
and electron microscopic radioautograms (LM and EM RAG) of the colonic and
caecal epithelia revealed that some of the nuclei of columnar epithelial cells were
labeled with 3H-leucine showing protein synthesis. The labeled cells
were located at the lower half of the crypts, and the labeling index (LI)
changed with the aging. A peak of the labeling index of the absorptive cells was
found at embryonic day 19, but decreased at the postnatal day 1 and then kept
an almost constant value until postnatal month 12. On the other hand, the LI of
the goblet cells showed the peak at embryonic day 19, then decreased gradually
with aging from postnatal day 1 and completely disappeared from postnatal month
1 onwards, while the basal granulated cells were located only in the base of
crypts and the significant difference of LI was not found from embryonic day 19
to postnatal month 12. However, the localizations of silver grains over the
mitochondria of these cells were not examined in these studies [1, 2, 3]. In the previous studies, we
observed the DNA synthesis in the mitochondria of columnar epithelial cells,
mainly the columnar absorptive cells, in 10 groups of litter mate mice, from
embryonic day 19, postnatal day 1, 3, 7, 14, and month 1, 6, 12 (year 1) and 24
(2 years), where the number of labeled cells were counted and the labeling
indices of these cells were analyzed.
On the other hand, in contrast to the
DNA synthesis in nuclei in various cells of colonic and cecal epithelial cells
in aging mice, we also found the silver grains due to DNA and RNA syntheses in
mitochondria of various cells such as the pancreatic acinar cells, hepatic
cells, adrenal cells or renal cells showing intramitochondrial DNA and RNA syntheses
[4, 5, 6, 7]. We later found that the activities of DNA and RNA syntheses in
mitochondria of various cells changed due to aging of individual animals [8, 9,
10, 11].
Thus, we have formerly concentrated to study
the intramitochondiral DNA and RNA as well as protein
synthesis in various cells of aging mice [12], especially in the liver that
contained many mitochondria [13]. We also found that the activities of DNA and
RNA syntheses in mitochondria of colonic epithelial cells changed due to aging
of individual animals [14,15]. To the contrary, this paper deals with the
intramitochondrial protein synthesis in colonic epithelial cells of aging ddY mice at various ages in 10 groups during development
and aging from prenatal embryo day 19 to postnatal 2 years at senescence.
MATERIALS AND
METHODS
1. The experimental animals
The colonic tissues were obtained from
10 groups of aging normal ddY strain mice, each
consisting of 3 litter mates of both sexes, total 30, from prenatal embryo day
19 to newborn postnatal day 1, 3, 7, 14, adult at month 1, 2, 6, 12 (year 1) to
month 24 (year 2). All the animals were housed under conventional conditions
and bred with normal diet (mouse chow Clea EC2, Clea Co., Tokyo, Japan) with access to water ad libitum in our laboratory. They were administered with 3H-leucine,
one of the amino-acid and the protein precursors, and the colonic tissues were
taken out, fixed and processed for electron microscopic radioautography. All
the procedures used in this study concerning the animal experiments were in
accordance with the guidelines of the animal research committee of Shinshu
University School of Medicine as well as the principles of laboratory animal
care in NIH publication No. 86-23 (revised 1985).
2. Procedures of microscopic
radioautography
All the animals were injected intraperitoneally with 3H-leucine (Amersham, England, specific activity 877 GBq/mM) in saline, at 9 a.m., one
hour before sacrifices. The dosage of injections was 370 KBq/gm
body weight. The animals were perfused at 10 a.m.,
one hour after the injection, via the left ventricles of the hearts with 0.1 M cacodylate-buffered 2.5% glutaraldehyde
under Nembutal (Abbott Laboratories, Chicago, ILL, USA) anesthesia. The distal
colon was taken out from each animal, excised into small tissue pieces of the colonic
tissues (size 1mm x 1mm x 1mm) which were immersed in the same fixative at
4˚C for 1 hr., followed by postfixation in 1%
osmium tetroxide in the same buffer at 4˚C for 1
hr., dehydrated in graded series of ethanol and acetone, and embedded in epoxy
resin Epok 812 (Oken,
Tokyo, Japan).
For light microscopic radioautography, semithin sections at 0.5µm thickness, thicker than
conventional ultrathin sections in order to shorten the exposure time for
radioautography, were cut in sequence on a Porter-Blum MT-2B ultramicrotome (Dupont-Sorvall,
Newtown, MA, USA) using glass knives. The sections were collected on collodion coated glass slides, coated with Konica NR-M2 radioautographic emulsion (Konica, Tokyo, Japan) by a
dipping method [5, 6, 7]. They were stored in dark boxes containing silica gel
(desiccant) at 4˚C for exposure. After the exposure for 2 months, the
specimens were processed for development in freshly prepared D-19 solution for
10 min at 16˚C in a water bath, rinsed in distilled water and dried in an
oven at 37˚C overnight, stained with toluidine
blue solution for 2 min and dried for light microscopy.
For electron microscopic
radioautography, semithin sections at 0.2µm
thickness, thicker than conventional ultrathin sections in order to shorten the
exposure time for radioautography, were cut in sequence on a Porter-Blum MT-2B ultramicrotome (Dupont-Sorvall,
Newtown, MA, USA) using glass knives. The sections were collected on collodion coated copper grid meshes (VECO, Eerbeek, Netherlands), coated with Konica NR-H2 radioautographic emulsion (Konica, Tokyo, Japan) by a
wire-loop method [5, 6, 7]. They were stored in dark boxes containing silica
gel (desiccant) at 4˚C for exposure. After the exposure for 10 months, the
specimens were processed for development in freshly prepared gold latensification solution for 30 sec at 16˚C and then
in fresh phenidon developer for 1 min at 16˚C in
a water bath, rinsed in distilled water and dried in an oven at 37˚C
overnight, stained with lead citrate solution for 3 min, coated with carbon for
electron microscopy. The electron microscopic (EM) radioautograms were examined
in a JEOL JEM-4000EX electron microscope
(JEOL, Tokyo, Japan) at accelerating voltages of 400 kV for observing
thick specimens.
3. Quantitative analysis of light
micrographs
For quantitative analysis of light
micrographs, twenty LM radioautograms showing cross sections of colonic
columnar absorptive cells from each group, based on the light microscopic
photographs taken after observation on at least 100 colonic epithelial cells
from respective animals were analyzed to calculate the total number of labeled
nuclei covered with silver grains by visual grain counting.
On the other hand, the numbers of silver
grains in the same area size as a nucleus outside cells were also calculated in
respective specimens as background fog, which resulted in less than 1 silver
grain (0.02/nuclear area), i.e., almost zero.
Therefore, the grain count in each specimen was not corrected with
background fog. From all the data thus obtained the averages and standard
deviations in respective aging groups were computed with a personal
computer (Macintosh type 8100/100, Apple
Computer, Tokyo, Japan). The data were stochastically analyzed using variance
and Student's t-test. The differences
were considered to be significant at P value <0.01.
4. Quantitative analysis of
electron micrographs
For quantitative analysis of electron
micrographs, twenty EM radioautograms showing cross sections of colonic columnar
absorptive cells from each group, based on the electron microscopic photographs
taken after observation on 100 colonic epithelial cells from respective animals
were analyzed to calculate the total number of mitochondria in each cell, and
the number of labeled mitochondria covered with silver grains by visual grain
counting.
On the other hand, the number of silver
grains in the same area size as a mitochondrion outside cells was also
calculated in respective specimens as background fog, which resulted in less
than 1 silver grain (0.02/mitochondrial area) almost zero. Therefore, the grain count in each specimen
was not corrected with background fog. From all the data thus obtained the
averages and standard deviations in respective aging groups were computed with
a personal computer (Macintosh type
8100/100, Apple Computer, Tokyo, Japan). The data were stochastically analyzed
using variance and Student's t-test. The
differences were considered to be significant at P value <0.01.
RESULTS
1. Morphological observations
The colonic tissues obtained from ddY strain mice at various ages from embryo day 19 to
postnatal month 24, consisted of 3 layers, i.e., the mucous layer, the muscular
layer and the serous membrane as observed by both light and electron microscopy.
The mucous layer of the colon does not form folds like the small intestines or
the last portion of the large intestine, i.e. the rectum. The colonic mucous
layer consists of the mucous membrane and the submucous
tissues. The former consists of the simple columnar epithelium forming
intestinal crypts but not such villi as in the small intestines, while the
latter consists of the connective tissues. Therefore, the colonic mucous
membrane has a smooth surface, that is lined by simple columnar epithelium with
a thin striated border. The intestinal crypts, or the glands of Lieberkuehn are
straight tubules, which attain a greater length in the colon than in the small
intestines. The colonic epithelial cells differ from the small intestines in
their greater abundance of goblet cells. The epithelial cells proliferate at
the bottom of the crypts where undifferentiated proliferating cells exists.
We have studied the macromolecular
synthesis of the colonic epithelial cells in the aging mice in 10 groups of
litter mates of both sexes, each 3, from embryonic day 19 to postnatal day 1,
3, 7, 14, month 1, 2, 6, 12 (year 1) and 24 (year 2), by means of light and
electron microscopic radioautography. We
first studied the DNA synthesis in the colon and caecum of aging mice from
embryonic day 19 to postnatal month 12 by using 3H-thymidine [1, 2,
3]. Light and electron microscopic
radioautograms (LM and EM RAG) of the colonic and caecal epithelia revealed
that some of the nuclei of columnar epithelial cells were labeled with 3H-thymidine
showing DNA synthesis. The labeled cells were located at the lower half of the
crypts, and the labeling index (LI) changed with the aging. A peak of the
labeling index of the absorptive cells was found at embryonic day 19, but
decreased at the postnatal day 1 and then kept an almost constant value until
postnatal month 12. On the other hand, the LI of the goblet cells showed the
peak at embryonic day 19, then decreased gradually with aging from postnatal
day 1 and completely disappeared from postnatal month 1 onwards, while the
basal granulated cells were located only in the base of crypts and the
significant difference of LI was not found from embryonic day 19 to postnatal
month 12. We also studied the localizations of silver grains over the
mitochondria of columnar epithelial cells, mainly the columnar absorptive
cells, in 10 groups of litter mate mice, from embryonic day 19, postnatal day
1, 3, 7, 14, and month 1, 6, 12 (year 1) and 24 (2 years) and the number of
labeled cells were counted and the labeling indices of these cells were
analyzed [14,15].
Among the epithelial cells covering the
colon, the columnar absorptive cells at the bottom of the crypts were analyzed
in this study.
2. Radioautographic
Observations
Observing electron microscopic
radioautograms of the columnar epithelial cells, the silver grains were found
over the nuclei as well as over the cytoplasm including mitochondria of some columnar
epithelial cells (Figs. 1-7), labeled with 3H-leucine, demonstrating
protein synthesis at respective aging stages from perinatal
stages at embryonic day 19 (Fig. 1), to postnatal day 1 (Fig. 2) and day 3 and
7 (Fig. 3), and day 14, to adult stage at month 1 (Fig. 4), month 2 and 6 (Fig.
5), and to senescent stage at month 12 (Fig. 6) and 24 (Fig. 7).
The localizations of silver grains over
the mitochondria were mainly on the mitochondrial matrices similarly to other
cells such as in the livers [13] or the adrenal glands [14] as reported
previously.
3. Quantitative Analysis
3.1. Number of mitochondria per
cell
Preliminary quantitative analysis on the
number of mitochondria in 10 columnar epithelial cells whose nuclei were
labeled with silver grains and other 10 cells whose nuclei were not labeled in
each aging group revealed that there was no significant difference between the
number of mitochondria and the labeling indices (P<0.01). Thus, the number
of mitochondria and the labeling indices were calculated regardless whether
their nuclei were labeled or not. The results obtained from the number of
mitochondria in columnar epithelial cells of respective animals in 10 aging
groups at perinatal and newborn stages, from prenatal
embryo day 19 to postnatal day 1, 3, 7, 14, and adult and senescent stages at
month 1, 2, 6, 12, and 24, seemed to show an gradual increase from the prenatal
day 19 to postnatal month 24.
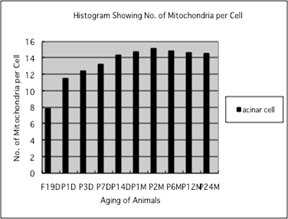
Counting the number of mitochondria per
cell at respective aging stages, it increased from prenatal embryo around 5.7/cell
in average, to 6.2 at postnatal day 1, to 7.5 at day 3, to 9.3 at day 7, to 10.1
at day 14, to 11.7 at month 1, to 11.8 at month 2, then slightly decreased to 11.2
at month 6, to 11.1 at month 12 and finally to 10.4 at month 24 as shown in
Fig. 7. All the data from embryonic day 19 to postnatal month 24, were stochastically
analyzed using variance and Student's t-test. The increases of mitochondrial
numbers in the colonic columnar epithelial cells from embryonic day 19 to adult
stage at postnatal month 2 were considered to be significant at P value
<0.01. However, the slight decrease at the senescent stage from month 6 to
24 were considered to be not significant at P value <0.01.
3.2. Mitochondrial protein
synthesis
The results of visual counting on the
number of mitochondria labeled with silver grains obtained from 10 columnar
epithelial cells of each animal labeled with 3H-leucine
demonstrating protein synthesis in 10 aging groups at perinatal
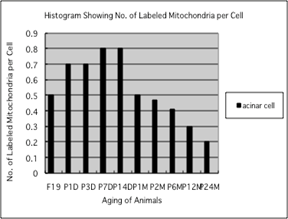
stages, from prenatal embryo day 19 (4.9/cell), postnatal day 1, 3, 7 and 14,
to adult stages at month 1, 3, and 6, 12 and 24, increased gradually to day 1 (5.6),
to day 3 (6.8) to day 7 (8.2) and day 14 (8.9), to month 1 (9.8), to month 2 (10.4),
reaching the maximum, then decreased gradually to month 6 (10.2), to month 12 (10.3)
and month 24 (9.8/cell) as shown in Fig. 9.
The data were stochastically analyzed using variance and Student's
t-test. The increases of the numbers of labeled mitochondria from embryo day 19
to postnatal month 2, were stochastically significant (P <0.01). However,
the decreases from month 2 to month 24 were not significant.
3.3. The labeling index
Finally, the labeling indices of
mitochondrial protein synthesis incorporating 3H-leucine in the colonic
columnar epithelial cells at respective aging stages were calculated from the
number of labeled mitochondria (Fig. 9) dividing by the number of total mitochondria
per cell (Fig. 8), which were plotted in Fig. 10.
The results showed that the labeling
indices increased from prenatal day 19 (85.9%) to postnatal newborn day 1 (90.3%),
to postnatal day 3 (90.7%), then gradually decreased to day 7 (88.1%), to day
14 (86.1 %), then again increased to to adult stages
at month 1 (88.1%), month 2 (88.9 %), month 6 (89.3%), month 12 (93.1%) and 24
(94.2%), reaching the maximum, as shown in Fig. 10. From the results, the increase of the
mitochondrial labeling indices in colonic columnar epithelial cells from embryo
day 19 to newborn postnatal day 3 and the decrease to postnatal day 7 and 14, as
well as the increase to month 1 to month 24 were stochastically significant (P
<0.01).
DISCUSSION
From the results obtained in the present
study on the colonic columnar epithelial cells of ddY
ageing mice at various ages in 10 groups from perinatal
stages at embryo day 19, to newborn day 1, 3, juvenile day 7, 14, and young
adult at postnatal month 1, 2, 6 as well as the senescent adult at postnatal
month 12 and 24, it was shown that intramitochondrial DNA synthesis was
observed in the colonic columnar epithelial cells of all the aging stages from
prenatal embryos to postnatal newborn, juvenile and young adult, senescent adult
stages and the number of mitochondria per cell showed increases due to ageing,
while the number of labeled mitochondria per cell and the labeling index showed
increases and decreases due to ageing. However, there was a discrepancy between
the increases and decreases of the number of total mitochondria and labeled
mitochondria that showed single increase and decrease and the two peaks of
increases in the labeling index. The inconsistency may be due to the difference
of the timing when the mitochondria synthesized protein at juvenile and young
stages from postnatal day 1 to month 2 when the number of mitochondria
increased rapidly. These results demonstrated that intramitochondrial protein
synthesis in the colonic epithelial cells revealed variations due to ageing of
individual animals depending upon the cellular activities at respective ageing
stages.
With regards the macromolecular
synthesis in various cells in various organs of experimental animals observed
by light and electron microscopic radioautography, it is well known that the
silver grains due to radiolabeled 3H-thymidine
demonstrate DNA synthesis [1, 4, 6, 10,
12-18]. The previous results obtained
from the studies on the hepatocytes of ageing mice by
light and electron microscopic radioautography revealed that silver grains
indicating DNA synthesis incorporating 3H-thymidine were observed
over the nuclei of some hepatocytes at perinatal stages from postnatal day 1 to day 14 and
decreased due to ageing [15-18]. Then, we lately observed the
intramitochondrial DNA synthesis in the various organs such as the livers [12,
13, 19-22] adreno-cortical [14, 23-26], adreno-medullary cells [14, 27, 28] and the pancreatic
acinar cells [93, 94], at various ages from fetal day 19 to postnatal newborn
day 1, 3, juvenile day 7, 14 and to adult month 1, 2, 6, 12 and 24. In the
present study, further data obtained from the colonic columnar epithelial cells
from prenatal to adult senescent animals at postnatal month 12 and 24 were
added.
On the other hand, we also studied the
numbers of silver grains showing nuclear RNA synthesis resulting from the
incorporations of 3H-uridine into mitochondria indicating
mitochondrial RNA synthesis demonstrated the silver grain localization over the
mitochondria independently from the nuclei whether the nuclei were labeled with
silver grains or not in the pancreatic acinar cells from prenatal embryo day 19
to postnatal month 24 during the development and ageing [95]. The numbers of
labeled mitochondria showing RNA synthesis as well as the labeling indices
increased from perinatal embryonic day to postnatal
newborn and juvenile stages at day 14, to adult postnatal month 1, 2, 6, reaching
the maxima, and then decreased to the senescent stages at month 12 and 24.
With regards DNA in mitochondria in
animal cells or plastids in plant cells, many studies have been reported in
various cells of various plants and animals since 1960s [29-34]. Most of these
authors observed DNA fibrils in mitochondria which were histochemically
extracted by DN’ase. Electron microscopic observation
of the DNA molecules isolated from the mitochondria revealed that they were
circular in shape, with a circumference of 5-6 µm [35]. It was calculated that
such a single molecule had a molecular weight of about 107 daltons [36].
Mitochondria of various cells also contained a DNA polymerase, which was
supposed to function in the replication of the mitochondrial DNA [37]. On the
other hand, the incorporations of 3H-thymidine into mitochondria
demonstrating DNA synthesis were observed by means of electron microscopic
radioautography in lower organism such as slime mold [38, 39], tetrahymena [40] or chicken fibroblasts in tissue culture
under abnormal conditions [41]. However, these authors used old-fashioned
developers consisting of methol and hydroquinone
(MQ-developer) that produced coarse spiral silver grains resulting in
inaccurate localization over cell organelles when observed by electron
microscopy. All of these authors showed photographs of electron radioautographs with large spiral-formed silver grains (2-3
µm in diameter) localizing not only over the mitochondria but also outside the
mitochondria. In order to obtain smaller silver grains, we first used elon-ascorbic acid developer after gold latensification
[7, 15], which produced comma-shaped smaller silver grains (0.4-0.8 µm in
diameter), then later we used phenidon developer
after gold latensification, producing dot-like
smaller silver grains (0.2-0.4 µm in diameter) localizing only inside the
mitochondria showing ultrahigh resolution of radioautograms [1, 12, 13, 42,
43]. These papers were the first that demonstrated intramitochondrial DNA
synthesis incorporating 3H-thymidine with accurate
intramitochondrial localization in avian and mammalian cells. With regards the
resolution of electron microscopic radioautography, on the other hand, many
authors discussed the sizes of silver grains under various conditions and
calculated various values of resolutions [8, 10, 44-46]. Those authors who used
the M-Q developers maintained the resolution to be 100-160 nm [44, 45], while
those authors who used the elon-ascorbic acid
developer [8, 10, 46] calculated it to be 25-50 nm. When we used phenidon developer at 16˚C for 1 min after gold latensification, we could produce very fine dot-shaped
silver grains and obtained the resolution around 25 nm [1, 12, 13, 42, 43, 46].
For the analysis of electron radioautographs, Salpeter et al. [40] proposed to use the half-distance and
very complicated calculations through which respective coarse spiral-shaped
silver grains were judged to be attributable to the radioactive source in a
certain territory within a resolution boundary circle. However, since we used phenidon
developer after gold latensification to produce very
fine dot-shaped silver grains, we judged only the silver grains which were
located in the mitochondria which were dot-shaped very fine ones to be
attributable to the mitochondria without any problem as was formerly discussed
[8, 10, 12, 13, 42, 43].
Then we also demonstrated
intramitochondrial DNA synthesis incorporating 3H-thymidine in some
other established cell lines originated from human being such as HeLa cells [8, 10] or mitochondrial fractions prepared from
in vivo mammalian cells such as rat and mouse [9, 11]. It was later commonly
found in various cells and tissues not only in vitro obtained from various
organs in vivo such as the cultured human HeLa cells
[47], cultured rat sarcoma cells [48], mouse liver and pancreas cells in vitro
[48, 50, 51], but also in vivo cells obtained from various organs such as the
salivary glands [52], the liver [53-64,91], the pancreas [65], the trachea
[66], the lung [67], the kidneys [68], the testis [69,70], the uterus [71,72],
the adrenal glands [73-75], the brains [76], and the retina [77-81] of mice,
rats and chickens. Thus, it is clear that all the cells in various organs of
various animals synthesize DNA not only in their nuclei but also in their
mitochondria.
The relationship between the
intramitochondrial DNA synthesis and cell cycle was formerly studied in
synchronized cells and it was clarified that the intramitochondrial DNA
synthesis was performed without nuclear involvement [8]. However, the relationship between the DNA
synthesis and the aging of individual animals and men has not yet been fully
clarified except a few papers published by Korr and
associates on mouse brain [82-85]. They reported both nuclear DNA repair,
measured as nuclear unscheduled DNA synthesis, and cytoplasmic
DNA synthesis labeled with 3H-thymidine in several types of cells in
brains such as pyramidal cells, Purkinje cells, granular cells, glial cells, endothelial cells, ependymal
cells, epithelial cells as observed by light microscopic radioautography using
paraffin sections. They observed silver
grains over cytoplasm of these cells by light microscopy and maintained that it
was reasonable to interpret these labeling as 3H-DNA outside the
nuclei, which theoretically belonged to mitochondrial DNA without observing the
mitochondria by electron microscopy.
From the results, they concluded that distinct types of neuronal cells
showed a decline of both unscheduled DNA and mitochondrial DNA syntheses with
age in contrast that other cell types, glial and
endothelial cells, did not show such age-related changes without counting the
number of mitochondria in respective cells nor counting the labeling indices at
respective aging stages. Thus, their results from the statistics obtained from
the cytoplasmic grain counting seems to be not
accurate without observing mitochondria directly. To the contrary, we had
studied DNA synthesis in the livers of aging mice [53-64] and clearly
demonstrated that the number of mitochondria in each hepatocytes,
especially mononucleate hepatocytes,
increased with the ages of animals from the perinatal
stages to adult and senescent stages, while the number of labeled mitochondria
and the labeling indices increased from the perinatal
stages, reaching a maximum at postnatal day 14, then decreased.
Our previous studies [59, 60] also clarified
that the DNA synthesis and cell proliferation by mitosis were the most active
in the nuclei of mononucleate hepatocytes
at the perinatal stages in contrast that binucleate cells were less active at the perinatal stage but the number of binucleate
hepatocytes increased at senescent stages and the
results suggest the possibility that the mitochondria in mononucleate
hepatocytes synthesized their DNA by themselves which
peaked at postnatal day 14 in accordance with the proliferation of mononucleate hepatocytes while binucleate hepatocytes increased
after the perinatal stage and did not divide but
remained binucleate keeping many mitochondria in
their cytoplasm which were more in number than mononucleate
hepatocytes at the senescent stage.
Thus, our previous papers were the first
which dealt with the relationship between the DNA synthesis and aging in hepatocytes of mice in vivo at various ages by means of
electron microscopic radioautography observing the small dot-like silver
grains, due to incorporations of 3H-thymidine, which exactly
localized inside the mitochondria.
Later we also studied intramitochondrial
DNA synthesis in adreno-cortical cells from prenatal
day 19 to postnatal day 1, 3, 9, 14, month 1, 2, 6, 12 and 24 (year 2) and
found that the numbers of mitochondria in 3 zones, glomerulosa,
fasciculate and reticularis, increased reaching the
maxima at postnatal month 2 and which kept continued until senescence up to 24
months (2 years). To the contrary, the numbers of labeled mitochondria and the
labeling indices increased to postnatal month 2, reaching the maxima, then
decreased to month 24 [23-28]. Later we also observed that the number of the pancreatic
acinar cells showing DNA synthesis in mitochondria and the labeling indices
increased to postnatal day 14, reaching the maxima, then decreased to month 24
[93, 94]. On the other hand, we also demonstrated the results from the RNA
synthesis in the livers and adrenal glands of ageing mice which also revealed
that an increase was observed by direct observation on mitochondria at electron
microscopic level and obtaining accurate mitochondrial number and labeling
indices in the hepatocytes and adreno-cortical
and adreno-medullary cells. In the present study, we
also demonstrated the RNA synthesis in the pancreatic acinar cells in 10 groups
of developing and aging mice. There was a discrepancy between our results from
the hepatocytes [59, 60], the adrenal cells [23-28], the
pancreatic acinar cells [93, 94] as well as the colonic columnar epithelial
cells at present and the results from the several types of cells in the brains
by Korr et al. [82-85]. The reason for this
difference might be due to the difference between the cell types (hepatocytes, adrenal cells, pancreatic acinar cells or
colonic columnar epithelial cells from our results and the brain cells from
their results) or the difference between the observation by light or electron
microscopy, i.e., direct observation of mitochondria by electron microscopy in
our results or light microscopy, i. e., indirect
observation of mitochondria without observing any mitochondria directly by Korr et al. [82-85].
Anyway, the results obtained from the
colonic epithelial cells of aging mice at present should form a part of special
cytochemistry [17] in cell biology, as well as a part
of special radioautographology [12], i.e., the
application of radioautography to the pancreas, as was recently reviewed by the
present author including recent results dealing with various organs [86-94]. We
expect that such special radioautographology and
special cytochemistry should be further developed in
all the organs in the future.
CONCLUSION
From the results obtained at present, it
was concluded that almost all the columnar epithelial cells in the colons of
mice at various ages, from prenatal embryo day 19 to postnatal newborn, day 1,
3, 7 and 14, and to postnatal month 1, 2, 6, 12 and 24, were labeled with
silver grains showing protein synthesis with 3H-leucine in their
mitochondria. Quantitative analysis on the number of mitochondria in colonic
columnar epithelial cells showed that they increased from prenatal embryo day
19 to postnatal day 1, 3, 7, 14, to month 1, month 2, reaching a maximum, then
slightly decreased to month 6, 12 and finally to month 24. Likewise, the number
of labeled mitochondria with 3H-leucine showing protein synthesis increased
from prenatal day 19 to postnatal day 1, 3, 7, 14, to month 1 and 2, reaching a
maximum, then slightly decreased to month 6, 12 and 24. To the contrary, the
labeling index increased from prenatal day 19 to postnatal day 1, reaching a
maximum, then decreased to day 3, 7, 14 and month 1, reaching another maximum, 2,
and decreased again to month 12 and 24.
These results demonstrated that the
number of mitochondria in the colonic epithelial cells increased from perinatal stages to postnatal month 2 and 6, keeping the
maximum up to month 24, while the activity of mitochondrial DNA synthesis
increased from prenatal to postnatal day 1, then decreased to postnatal month 1
and again increased to month 6, reaching the maximum, then decreased to month
24 due to ageing of animals.
ACKNOWLEDGEMENTS
This study was carried out after the
author retired from Shinshu University School of Medicine and was not
supported by any research grants from any foundations or national government
except a small support from a private school, Shinshu Institute of Alternative
Medicine and Welfare, where the author was working. The author thanks Dr.
Kiyokazu Kametani, Technical Official, Research
Center for Instrumental Analysis, Shinshu University, for his technical
assistance during the course of this study.
REFERENCES
[1] Nagata, T., Usuda, N.
[1985] Image processing of electron microscopic radioaautograms
in clinical electron microscopy. J. Clin. Electron Microsc. 18: 5-6.
[2] Nagata, T., Usuda N,,Ma, H. [1986] Electron microscopic radioautography of
nucleic acid synthesis in pancreatic acinar cells of prenatal and postnatal
aging mice. Proc. 11th Internat. Cong. Electron Microsc., Kyoto, Japan, Vol. 3, pp. 2281-2282.
[3] Nagata, T., Usuda, N.,
Ma, H. [1986] Application of high voltage electron microscopy to histochemistry of whole mount preparations of cultured
cells or thick sections from embedded tissues. Proc. 11th Internat.
Cong. Electron Micrsco. Kyoto, Japan, pp.1183-1184.
[4] Nagata, T., Usuda, N.,
Ma, H. [1986] Electron microscopic radioautograpahy
of nucleic acid synthesis in pancreatic acinar cells of prenatal and postnatal
aging mice. Proc. 11th Internat. Cong. Electron Micrsco. Kyoto, Japan, pp.2281-2282.
[5] Nagata, T. [1992] Radiolabeling
of soluble and insoluble compounds as demonstrated by light and electron
microscopy. In, Recent Advances in Cellular and Molecular Biology. Vol. 6
Molecular Biology of Pyrimidines, DNA, peroxisomes, organelles and cell movements. Wegmann, R. J. and Wegmann, M. A.
eds., Peeters Press, Leuven, Belgium, pp. 9-21.
[6] Nagata, T. [1993] Quantitative analysis of histochemical reactions: image analysis of light and
electron microscopic radioautograms. Acta Histochem. Cytochem. 26: 281-191.
[7] Nagata, T. [1993] Quantitative light and
electron microscopic radioautographic studies on
macromolecular synthesis in several organs of prenatal and postnatal aging
mice. Chinese J. Histochem. Cytochem.
2: 106-108.
[8] Nagata, T. [1972] Radioautographic
study on intramitochondiral nucleic acid synthesis:
Its relationship to the cell cycle in cultivated cells. Proc. 4th Internat. Cong. Histochem. Cytochem. Kyoto, Japan, 1: 223-224.
[9] Nagata, T. [1974] Electron microscopic
radioautography of intramitochondrial nucleic acid synthesis in mammalian cells
in vitro. Proc. 8th Internat. Cong.
Electron Microsc., Canberra, Australia, 2: 346-347.
[10] Nagata, T. [1972] Electron microscopic
radioautography of RNA synthesis of HeLa cells in
culture. Histochemie. 32: 163-170.
[11] Nagata, T., Iwadare,
N., Murata, F. [1976] Mitochondiral and nucleolar RNA synthesis as revealed by electron microscopic
radioatography. Proc. 5th Internat. Cong. Histochem. Cytochem. Bucharest, Romania, 1: 242-243.
[12] Nagata, T. [2002] Radioautographology,
General and Special. In, Prog. Histochem.
Cytochem., Graumann, W.
ed., Urban-Fischer, Jena, Germany, 32(2): pp. 57-228.
[13] Nagata, T. [2010] Macromolecular synthesis in
the livers of aging mice as revealed by electron microscopic radioautography.
In, Prog. Histochem. Cytochem., Sasse, D., Schumacher,
U., eds., Eslevier, Jena, Germany, 45(1): pp.
1-80.
[14] Nagata,
T.: DNA synthesis in the colonic cells of aging mice as revealed by microscopic
radioautography. J. Global Issue Solutions, in press, 2012
[15]
Nagata T.: RNA synthesis in the colonic epithelial cells of aging mice as revealed
by microscopic radioautography. British
Journal of Medicine and Medical Research (BJMMR), in press, 2012
[16] Nagata, T.
[2011] Macromolecular synthesis in mitochondria in adrenal glands of mice as
observed by electron microscopy. In, DNA Microarrays, Synthesis and Synthetic
DNA, Nova Science Publishers, NY, USA, p. 76-162, 2011.
[17] Nagata, T., Shibata, O, Nawa,
T. [1967] Incorporation of tritiated thymidine into mitochondrial DNA of the liver and kidney
cells of chickens and mice in tissue culture. Histochemie.
10: 305-308.
[18] Nagata, T. [1999] Aging changes of
macromolecular synthesis in various organ systems as observed by microscopic radioautogtraphy. Methods Find. Exp. Clin.
Pharmacol. 21: 683-706.
[19] Nagata, T. [2001] Chapter 2, Special cytochemistry in cell biology. In, Jeon,
K. W. ed., International Review of Cytology, Vol. 211, Academic Press, N. Y.,
USA, pp. 33-154.
[20] Nagata, T. [2003] Light and electron
microscopic radioautographic studies on
macromolecular synthesis in amitotic hepatocytes in
aging mice. Cell. Mol. Biol. 49: 591-611.
[21] Nagata T.
[2007] Macromolecular synthesis in hepatocyte
mitochondria of aging mice as revealed by electron microscopic radioautography.
I: Nucleic acid synthesis. In, Modern Research and Educational Topics in
Microscopy. Mendez-Vilas, A., Diaz, J. eds., Formatex
Micrscopy Series No. 3, Formatex,
Badajoz, Spain, Vol. 1: pp. 245-258.
[22] Nagata T.
[2007] Macromolecular synthesis in hepatocyte
mitochondria of aging mice as revealed by electron microscopic radioautography.
II: Protein synthesis. In, Modern Research and Educational Topics in
Microscopy. Mendez-Vilas, A. and Diaz, J. eds., Formatex
Micrscopy Series No. 3, Formatex,
Badajoz, Spain, Vol. 1: pp. 259-271.
[23] Nagata, T. [2009] Sexual difference between the
macromolecular synthesis of hepatocyte mitochondria
in male and female mice in aging as revealed by electron microscopic
radioautography. Chapter 22. In, Women and Aging: New Research, H. T. Bennninghouse, A. D. Rosset, eds.
Nova Biomed. Books, New York, USA, pp. 461-487.
[24] Nagata, T. [2009] Protein synthesis in hepatocytes of mice as revealed by electron microscopic
radioautography. In, Protein Biosynthesis. Esterhouse,
T. E. and Petrinos, L. B., eds., Nova Biomed. Books,
New York, USA, pp.133-161.
[25] Nagata, T. [2008] Electron microscopic radioautographic study on mitochondrial DNA synthesis in
adrenal cortical cells of developing mice. J. Cell.Tis.
Res. 8: 1303-1312.
[26] Nagata T.
[2008] Electron microscopic radioautographic study on
mitochondrial DNA synthesis in adrenal cortical cells of developing and aging
mice. The Sci. World J. 8: 683-97.
[27] Nagata,
T. [2009] Electron microscopic radioautographic
study on mitochondrial DNA synthesis in adreno-cortical
cells of aging ddY mice. Bull. Shinshu Inst. Alternat. Med. Welfare 4:
51-66.
[28] Nagata T.
[2010] Electron microscopic radioautographic study on
mitochondrial RNA synthesis in adrenocortical cells
of aging mice. Open Anat. J. 2: 91-97.
[29] Nagata, T. [2009] Electron microscopic radioautographic study on mitochondrial DNA synthesis in
adrenal medullary cells of developing and aging mice.
J. Cell Tissue Res. 9: 1793-1802.
[30] Nagata, T. [2009] Electron
microscopic radioautographic study on DNA synthesis
of mitochondria in adrenal medullary cells of aging
mice. Open Anat. J. 1: 14-24.
[31] Nass, S., Nass, M. M. K. [1963] Intramitochondrial fibers with DNA
characteristics. I. Fixation and electron staining reactions. II. Enzymatic and other hydrolytic
treatments. J. Cell Biol. 19: 593-629.
[32] Gibor, A., Granick, S. [1964] Plastids and mitochondria: Inheritable
system. Science 145: 890-897.
[33] Gahan, P. B., Chayen, J. [1965] Cytoplasmic deoxyribonucleic acid. Internat.
Rev. Cytol. 18: 223-247.
[34] Nass, M. M. K. (1966)
The circularity of mitochondrial DNA. Proc. Nat. Acad. Sci. U.S. 56:1215-1222.
[35] van Bruggen, E. F.
J., Borst, P., Ruttenberg,
G. J. C. M., Gruber, M., Kroon, A. M. [1966] Circular
mitochondrial DNA. Biochim. Biophys.
Acta, 119: 437-439.
[36] Sinclair, J. H., Stevens, B. J. [1966] Circular
DNA filaments from mouse mitochondria. Proc. Nat. Acad. Sci. U.S., 56: 508-514.
[37] Schatz, G. [1970] Biogenesis of mitochondria.
In, Membranes of Mitochondria and Chloroplasts. Racker
E, ed., Van Nostrand-Reinhold, New York, USA, pp.
251-314.
[38] Guttes, E., Guttes, S. [1964] Thymidine
incorporation by mitochondria in Physarum polycephalum. Science 145:1057-1058.
[39] Schuster, F. L. [1965] A deoxyribose
nucleic acid component in mitochondria of Didymium nigirpes,
a slime mold. Exp. Cell Res. 39: 329-345.
[40]
Stone, G. E., Miller, O. L. Jr. [1965] A stable mitochondrial DNA in Tetrahymena puriformis. Exp.
Zool. 159: 33-37.
[41] Chévremont, M. [1963] Cytoplasmic
deoxyribonucleic acids: Their mitochondrial localization and synthesis in
somatic cells under experimental conditions and during the normal cell cycle in
relation to the preparation for mitosis. Cell Growth and Cell Division.
Symposia of the Internat. Soc. for Cell Biol. Vol. 2,
Harris R. J. C., ed., Academic Press, New York, USA, p. 323-333.
[42] Salpeter, M. M., Bachmann,
L., Salpeter, E. E. [1969] Resolution in electron
microscope radioautography. J. Cell Biol. 44: 1-20.
[43] Nadler, N. J. [1971] The interpretation of
grain counts in electron microscope radioautography. J. Cell Biol. 49: 377-382.
[44] Nagata, T. [1996] Techniques and application of
electron microscopic radioautography. J. Electron Microsc.
45: 258-274.
[45] Nagata, T. [1997] Techniques and application of
microscopic radioautography. Histol. Histopathol. 12: 1091-1124.
[46]. Uchida, K., Mizuhira,
V. [1971] Electron microscope autoradiography with special reference to the
problem of resolution. Arch. Histol. Jap. 31:
291-320.
[47] Murata, F., Yoshida, K., Ohno,
S., Nagata, T. [1979] Electron microscopic radioautography using a combination
of phenidon developer and domestic emulsion. Acta Histochem. Cytochem. 12: 443-450.
[48] Nagata, T. [1984] Electron microscopic
observation of target cells previously observed by phase-contrast microscopy:
Electron microscopic radioautography of laser beam irradiated cultured cells.
J. Clin. Electron Microsc.
17: 589-590.
[49] Nagata, T., Murata, F., Yoshida, K., Ohno, S., Iwadare, N. [1977b]
Whole mount radioautography of cultured cells as observed by high voltage
electron microscopy. Proc. 5th Internat. Conf. High Voltage
Electron Microsc. Kyoto, Japan, pp. 347-350.
[50] Nagata, T., Iwadare,
N., Murata, F. [1977] Electron microscopic radioautography of nucleic acid
synthesis in cultured cells treated with several carcinogens. Acta Pharmacol. Toxicol. 41: 64-65.
[51] Nagata, T., Murata, F. [1977] Electron
microscopic dry-mounting radioautography for diffusible compounds by means of ultracryotomy. Histochemistry 54:
75-82.
[52] Nagata, T, Ohno, S.,
Murata, F. [1977] Electron microscopic dry-mounting radioautography for soluble
compounds. Acta Pharmacol. Toxicol. 41: 62-63.
[53] Nagata, T., Nawa, T.,
Yokota, S. [1969] A new technique for electron microscopic radioautography of
soluble compouds. Histochemie
18: 211-249.
[54] Nagata, T., Ito, M., Chen, S. [2000] Aging changes
of DNA synthesis in the submandibular glands of mice
as observed by light and electron microscopic radioautography. Ann. Microsc. 1: 13-22.
[55] Nagata, T., Ohno, S.,
Kawahara, I., Yamabayashi, S., Fujii,
Y., Murata, F. [1979] Light and electron microscopic radioautography of nucleic
acid synthesis in mitochondria and peroxisomes of rat
hepatic cells during and after DEHP administration. Acta
Histochem. Cytochem. 16:
610-611.
[56] Nagata, T., Ohno, S.,
Yoshida, K., Murata, F. [1982] Nucleic acid synthesis in proliferating peroxisomes of rat liver as revealed by electron microscopical radioautography. Histochem.
J. 14: 197-204.
[57] Nagata, T., Fujii, Y.
and Usuda, N. [1982] Demonstration of extranuclear nucleic acid synthesis in mammalian cells
under experimental conditions by electron microscopic radioautography. Proc. 10th Internat.
Cong. Electron Microsc., Hamburg, Germany, Vol. 2,
pp. 305-306.
[58] Ma, H., Nagata, T. [1988] Studies on DNA
synthesis of aging mice by means of electron microscopic radioautography. J. Clin. Electron Microsc. 21:
335-343.
[59] Ma, H., Nagata, T. [1988] Electron microscopic radioautographic study of DNA synthesis in the livers of
aging mice. J. Clin. Electron Microsc. 21: 715-716.
[60] Ma, H., Gao, F., Sun,
L., Jin, C., Nagata, T. [1994] Electron microscopic radioautographic
study on the synthesis of DNA, RNA and protein in the livers of aging mice.
Med. Electron Microsc. 27: 349-351.
[61] Nagata, T. [2003] Light and electron
microscopic radioautographic studies on macromolecular
synthesis in amitotic hepatocytes of aging mice.
Cell. Mol. Biol. 49: 591-611.
[62] Nagata, T. and Ma, H. [2005] Electron
microscopic radioautographic study on mitochondrial
DNA synthesis in hepatocytes of aging mouse. Ann. Microsc. 5: 4-18.
[63] Nagata, T. [2006] Electron microscopic radioautographic study on protein synthesis in hepatocyte mitochondria of developing mice. Ann. Microsc. 6:
42-54.
[64] Nagata, T. [2007] Electron microscopic radioautographic study on macromolecular synthesis in hepatocyte mitochondria of aging mouse. J. Cell Tissue Res.
7: 1019-1029.
[65] Nagata, T. [2007] Electron microscopic radioautographic study on nucleic acids synthesis in hepatocyte mitochondria of developing mice. Trends Cell Mol. Biol., 2: 19-33.
[66] Nagata, T. [2007] Electron microscopic radioautographic study on protein synthesis in mitochondria
of binucleate hepatocytes
in aging mice. The Scientific World J. 7: 1008-1023.
[67] Nagata, T., Usuda N,
Ma, H. [1986] Electron microscopic radioautography of nucleic acid synthesis in
pancreatic acinar cells of prenatal and postnatal aging mice. Proc. 11th Internat. Cong. Electron Microsc.,
Kyoto, Japan, Vol. 3, pp. 2281-2282.
[68] Sun, L., Gao, F.,
Jin, C., Nagata, T. [1997] DNA synthesis in the trachea of aging mice by light
and electron microscopic radioautography. Acta Histochem. Cytochem. 30: 211-220.
[69] Nagata, T., Sun, L. [2007] Electron Microscopic
Radioautographic Study on Mitochondrial DNA and RNA
Syntheses in Pulmonary Cells of Aging Mice.
Ann. Microsc.
7: 36-59.
[70] Hanai, T.. Nagata, T.
[1994] Electron microscopic radioautographic study on
nucleic acid synthesis in perinatal mouse kidney
tissue. Med. Electron Microsc. 27: 355-357.
[71] Gao, F., Ma, H., Sun,
L., Jin, C., Nagata T [1994] Electron microscopic radioautographic
study on the nucleic acid and protein synthesis in the aging mouse testis. Med. Electron Microsc.
27: 360-362.
[72] Gao, F., Chen, S.,
Sun, L., Kang, W., Wang, Z., Nagata, T. [1995] Radioautographic
study of the macromolecular synthesis of Leydig cells
in aging mouse testis. Cell. Mol. Biol. 41: 145-150.
[73] Yamada, A.T., Nagata, T. [1992] Light and
electron microscopic radioautography of DNA
synthesis in the endometria of pregnant ovariectomized mice during activation of implantation
window. Cell. Mol. Biol. 38: 763-774.
[74] Yamada, A. T., Nagata, T. [1993] Light and electron microscopic radioautographic studies on the RNA synthesis of peri-implanting
pregnant mouse uterus during activation of receptivity for blastocyst
implantation. Cell. Mol. Biol. 39: 221-233.
[75] Ito, M., Nagata, T. [1996] Electron microscopic
radioautographic study on DNA synthesis and the ultrastructure of the adrenal gland in aging mice. Med. Electron Microsc.
29: 145-152.
[76] Ito, M. [1996] Radioautographic
studies on aging changes of DNA synthesis and the ultrastructural
development of mouse adrenal gland.
Cell. Mol. Biol. 42: 279-292.
[77] Nagata, T. [2008] Electron microscopic radioautographic study on mitochondrial DNA synthesis in
adrenal cortical cells of developing mice. J. Cell.Tis.
Res. 8: 1303-1312.
[78] Cui, H., Gao, F., Ma,
H., Nagata, T. [1996] Study on DNA synthesis of cellular elements in the
cerebella of aging mice by light and electron microscopic radioautography. Proc.
4th China-Japan Joint Histochem. Cytochem.
Symp., Chongqing Publishing House, Chongqing, China,
pp. 111-112.
[79] Gunarso, W. [1984] Radioautographic studies on the nucleic acid synthesis in
the retina of chicken embryo II. Electron microscopic radioautography. Shinshu
Med. J. 32: 241-248.
[80] Gunarso, W., Gao, F., Cui, H., Ma, H., Nagata, T. [1996] A light and
electron microscopic radioautographic study on RNA
synthesis in the retina of chick embryo. Acta Histochem. 98: 300-322.
[81] Gunarso, W., Gao, F., Nagata, T. [1997] Development and DNA synthesis in
the retina of chick embryo observed by light and electron microscopic
radioautography. Cell. Mol. Biol. 43: 189-201.
[82] Kong, Y., Nagata, T. [1994] Electron
microscopic radioautographic study on nucleic acid
synthesis of perinatal mouse retina. Med. Electron Microsc.
27: 366-368.
[83] Nagata, T. [2006] Aging changes of
macromolecular synthesis in the avian and mammalian eyes as revealed by
microscopic radioautography. Ann. Rev. Biomed. Sci. 8: 33-67.
[84] Korr, H., Phillipi, V., Helg, C., Schiefer, J., Graeber, M. B., Kreutzberg, G. W. [1997] Unscheduled DNA synthesis and
mitochondrial DNA synthetic rate following injuring of the facial nerve. Acta Neuropathol. 94: 557-566.
[85] Korr, H., Kurz, C., Seidler, T. O., Sommer, D., Schmitz, C. [1998] Mitochondrial DNA synthesis
studied autoradiographically in various cell types in
vivo. Braz. J. Med. Biol. Res. 31: 289-298.
[86] Schmitz, C., Axmacher,
B., Zunker, U., Korr, H.
[1999] Age related changes of DNA repair and mitochondrial DNA synthesis in the
mouse brain. Acta Neuropathol.
97: 71-81.
[87] Schmitz, C., Materne,
S., Korr, H. [1999] Cell-type-specific differences in
age-related changes of DNA repair in the mouse brain - Molecular basis for a new
approach to understand the selective neuronal vulnerability in Alzheimer’s
disease. J. Alzheimer’s Disease 1: 387-407.
[88] Nagata, T. [2009] Recent studies on
macromolecular synthesis labeled with 3H-thymidine in various organs
as revealed by electron microscopic radioautography. Cur. Radiopharm. 2: 118-1128.
[89] Nagata, T. [2009] Electron microscopic radioatuographic studies on macromolecular synthesis in
mitochondria of various cells. 18EMSM
Conference Proc. 9th Asia-Pacific Microscopy Conference (APMC9), Kuala Lumpur,
Malaysia, pp. 48-50.
[90] Nagata,
T. [2009] Electron
microscopic radioautographic studies on
macromolecular synthesis in mitochondria of animal cells in aging. Ann. Rev.
Biomed. Sci. 11: 1-17.
[91] Nagata, T. [2009] Electron microscopic
radioautographic studies on macromoleclular
synthesis in mitochondria of some organs in aging animals. Bull. Shinshu Inst. Alternat.
Med. Welfare 4: 15-38.
[92] Nagata, T.
[2010] Electron microscopic radioautographic studies
on macromolecular synthesis in mitochondria of animal cells in
aging. Ann. Rev. Biomed. Sci. 12: 1-29.
[93] Nagata, T. [2010] Macromolecular
synthesis in the livers of aging mice as revealed by electron microscopic
radioautography. In, Progress in Histochemistry and Cytochemistry. Vol.
45, No. 1, pp. 1-80, Elsevier, Jena, New
York, 2010
[94] Nagata, T. [2011] DNA synthesis in the
pancreatic acinar cells of aging mice as revealed by electron microscopic
radioautography. IIOABJ (Institute of Integrative Omics and
Applied Biotechnology) 2: 31-39, 2011
[95]
Nagata, T. [2012] RNA synthesis in the pancreatic cinar
cells of aging mice as revealed by electron microscopic radioautography. Curr. Radiopharm. 5: 5-14, 2012.
EXPLANATION OF FIGURES
Fig: 1. Electron
microscopic radioautograms (EMRAG) of the undifferentiated columnar epithelial
cells, labeled with 3H-leucine, demonstrating protein synthesis at
embryonic day 19. The silver grains were found over the nuclei as well as over
the cytoplasm including mitochondria of some columnar epitheilal
cells. x2,000.
Fig: 2. EM RAG of the
differentiated columnar epithelial cells of a postnatal day 1 mouse labeled
with 3H-leucine, showing silver grains over the nuclei and
mitochondria. x3,000.
Fig: 3. EM RAG of several
columnar epithelial cells of a postnatal day 7 mouse labeled with 3H-leucine,
showing silver
grains over the nuclei and mitochondria.
x6,000.
Fig. 4. EM RAG of several
columnar epithelial cells including a few goblet cells of a postnatal month 1
mouse labeled with 3H-leucine, showing silver
grains over the nuclei and mitochondria.
x6,000.
Fig. 5. EM RAG of several
columnar epithelial cells including a few goblet cells of a postnatal month 6
mouse labeled with 3H-leucine, showing silver
grains over the nuclei and mitochondria.
x6,000.
Fig. 6. EM RAG of several
columnar epithelial cells of a postnatal month 12 mouse labeled with 3H-leucine,
showing silver
grains over the nuclei and mitochondria.
x6,000.
Fig. 7. EM RAG of several
columnar epithelial cells of a postnatal month 24 mouse labeled with 3H-leucine,
showing silver
grains over the nuclei and mitochondria.
x6,000.
Fig. 8. Histogram showing
the numbers of mitochondria per cell in respective aging groups.
Fig. 9. Histogram showing
the numbers of labeled mitochondria per cell in respective aging groups labeled
with 3H-leucine.
Fig. 10. Histogram showing
the average labeling indices in respective aging groups labeled with 3H-leucine.
[ BWW Society Home Page ]
© 2014 The Bibliotheque: World Wide Society