Characterization of Polyaniline Synthesized in Presence of
Kosh Prasad Neupane and Dr. Jin-Wook Ha
Department of Chemical Engineering, Soonchunhyang University, Asan, Korea.
ABSTRACT
VA-044 [2,2’-azobis{2-(2-imidazolin-2-yl)propane}dihydrochloride] initiated polymerization of aniline in acidic (Toluene sulfonic acid and HBr; and H2SO4) aqueous medium using support polymer (polyvinylpyrrolidone)(PVP) have been synthesized by chemical polymerization. Well dispersing and long time durable without particles aggregation (in case of H2SO4 doped); and TSA and HBr doped PANi were characterized by UV-VIS, FTIR-Spectroscopy and Scanning Electron Microscopy. The UV-VIS broad bands of PANi salt at about 350 nm and 800 nm indicate the presence of benzoid and quinoid rings. Scanning electron microscopy (SEM) study reveals a significant morphology change in PANi/PVP composite with VA-044.
Keywords : Conducting Polyaniline, VA-044, Dispersions
1. INTRODUCTION
Polyaniline (PANi), nowadays, is the most attracting conducting polymer because of its good environmental stability with excellent electrical, magnetic and optical properties. The important applications of PANi include secondary batteries, electromagnetic interference shielding, molecular sensors, non-linear optical devices, electrochromic displays and macroelectronic devices [1], anti-corrosion coatings [2], ion-exchanger for water purification especially for softening drinking water [3], welding (joining) of thermoplasics and thermosets [4]. Emeraldine salt (half oxidized PANi, y=0.5) is the most conductive form of PANi, which can be obtained by protonation of emeraldine base and oxidation of leucoemeraldine base as shown in Figure1. Besides the emeraldine, leucoemeraldine (fully reduced PANI, y=1), and pernigraniline (fully oxidizes form, y=0) [9]; nigraniline (75% oxidized form, y=0.25)[10] has also been reported. Soluble polypyrrole and soluble polyaniline have been introduced recently [5-7]. Good stability is essential for the characterization and application of polymers. Polyaniline is insoluble in common solvents because of stiffness of chain and inerchain interaction. Structural modification of PANi by incorporation of flexible alkyl chains into PANi through an N-alkylation method with leucoemeraldine base is another successful approach towards solubility of PANi in common organic solvents [8].
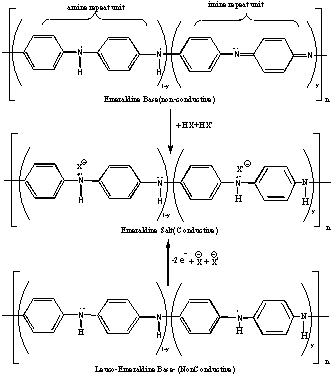
Figure 1. Structure of different forms of Polyaniline.
In the present work, we focus on the chemical synthesis of PANi using Azo-initiator VA-044 [2,2’-azobis{2-(2’-imidazolin-2-yl)propane}dihydrochloride] which decomposes at higher temperature (half life at 44 o C is 10 hrs) with liberation of molecular nitrogen and free-radicals as shown in Figure 2; and initiates free-radical polymerization; polyvinylpyrrolidone (PVP) as a steric stabilizer, ammonium peroxodisulphate (APS) as oxidant. The structure and properties thus obtained PANi were investigated.
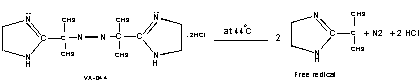
Figure 2. Structure and decomposition of VA-044.
2. EXPERIMENTAL
2.1. Materials
Aniline (Guaranteed Reagent) was purchased from Junsei Chemical Co. Ltd, Japan, VA-044 [2,2’-Azobis{2-(2-imidazolin-2-yl)propane}dihydrochloride] was purchased from Wako Pure Chemical Industries Ltd, Japan, ammonium peroxodisulphate (APS,,98.5%), Toluene sulfonic acid (TSA)(98.5%, ACS reagent grade), HBr (48%) and Polyvinylpyrrolidone (PVP) were purchased from Aldrich Chemical Company. These reagents were used without further purification.
2.2. Synthesis
2.2.1. Polyaniline/PVP/VA-044 composite
0.93gm (0.01 mole) of aniline was dissolved in aqueous acid or acid mixture (dopants). Then 0.5 gm of initiator followed by 1.0gm of PVP were added to the beaker containing stirring mixture of aniline monomer and dopant(s). The reaction temperature, compositions of all reactions are shown in Table 1. The reaction mixture was transferred into three-naked reaction flask (100 ml) and was stirred for 24 hrs at mentioned temperature. 2.28 gm APS oxidant (0.01 mole, same in all cases) was dissolved in 30 ml mixture of TSA and HBr (equivolume) for experiments from 1 to 3 and in water to make 20 ml solution for experiments 4 and 5. The oxidant solution was added to the stirring solution (1drop/sec).
Table 1. Synthesis of PANI composites in different reaction compositions
|
Exp. No.* |
Aniline (gm) |
Dopants
|
VA-044
|
PVP |
Rxn.Temp ( 0 C) |
Color before oxidation |
|
1. |
0.93 |
15ml 1M TSA +15ml 1M HBr |
- |
1.0 gm |
50 |
colorless |
|
2. |
0.93 |
15 ml 1M TSA +15 ml 1M HBr |
0.5 gm |
1.0 gm |
44 |
brown |
|
3. |
0.93 |
15 ml 1M TSA + 15 ml 1M HBr |
0.5 gm |
- |
44 |
brown |
|
4
|
0.93
|
20 ml 1 M H2SO4
|
0.5 gm
|
1.0 gm
|
44
|
brown
|
|
5. |
0.93 |
20 ml 1 M H2SO4
|
- |
1.0 gm |
44 |
brown |
* indicates the Experiment No. and also Sample No.
2.2.2. Coating of PVC sheet and Measurement of Surface Resistivity
The well-dispersed composite solutions was dropped on thin PET sheet cleaned by methanol and was coated by moving and pressing scroll bar in one direction. The coated film was dried in oven (~80 o C) for 5 minutes and surface resistivity was measured by SM-8220 (DKK.TOA Co., Japan) instrument.
2.2.3. Casting of PANi/PVP/VA044 composite film and dedoping
The prepared composite was poured over glass slide and allowed to dry in dust free environment for 1 day. The film of glass was dipped on concentrated ammonia solution for 12 hours, washed several times by distilled water and dried in drying oven at 80 o C for 24 hours. The dried powder sample was then submitted for FT-IR and SEM analysis.
2.3. Characterization
2.3.1. UV-VIS Spectra
The electronic spectra of PANi composite dispersions without any purification were recorded in UV-VIS spectrophotometer (JASCO-550).
2.3.1. FT-IR Spectra
FT-IR spectra of H2SO4 doped followed by NH4OH dedoped PANi/PVP and PANi/PVP/VA044 composites were taken using a FT-IR spectrometer (BIO-RAD Merlin) employing KBr disc method.
2.3.2. Scanning Electron Microscopy (SEM)
Surface morphology of PANi/PVP composites with and without VA-044 was taken by using JEOL JSM-5310 electron microscope at 15 kV (magnification 3500).
3. RESULTS AND DISCUSSION
3.1.Mechanism of Polymerization
The possible mechanism of polymerization is shown in Scheme I. The key of our investigation is thus obtained free radical attacks on anilinium ion and hence polymerization proceeds. It is believed that the free radical may serve two important other functions: firstly, it may prevent the formation of high molecular weight polyaniline by impeding the one or often two end of polymerization of polymer chain, secondly, the hydrogen atom of N-atom forms H-bond with O-atom of solvent which causes well dispersion.
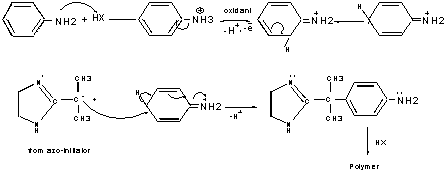
Scheme I. Proposed mechanism of Polymarization.
3.2. Morphology and Surface Resistivity
The nature of particles of PANi composites and the surface resistivity of PET film coated by polyaniline are listed in Table 2. To obtain well dispersion in aqueous solution was the main goal of the present work. PANi/PVP/VA-044 composite is more stable than PANi/PVP composites. This may be, of course, due to H-bonding between (i) N-atom of VA-044 bonded with PANi chain and –NH- of another PANi chain or H-of protic solvent (ii) –NH groups of VA-044 attached with PANi chain and O-atom of PVP or solvent containing O-atoms (iii) –NH group of PANi chain and O-atom of PVP or solvent (eg. water, ethanol etc). However, the macroscopic precipitation of PANi/PVP is disappeared on stirring. Moreover, the PANi doped by H2SO4 is more dispersed than PANi doped by TSA or TSA and HBr. This is because two O-atoms of H2SO4 may form H-bond with –NH- group of PANi or H-atom of solvent.
Table 2. Particles Nature and observed Surface resistivity of PANi synthesized in different condition
|
Sample No. |
Color of PANi after oxidation |
Particles Nature |
Surface Resistivity |
|
PANi-1. |
Blackish green |
Larger |
8 Ohm/square |
|
PANi-2.
|
Grass green
|
Smaller, aggregated after long time |
4.5 to 5 Ohm/square
|
|
PANi-3. |
Forest green |
Larger |
6.5 to 7 Ohm/square |
|
PANi-4 |
Deep green |
Particles are not seen by naked eye, no aggregation |
4 to 5 Ohm/square |
|
PANi-5 |
Deep green |
Particles are seen by naked eye, aggregated after long time |
4.5 Ohm/square |
3.3. UV-Vis Spectroscopy
The UV-Vis spectra of PANi were taken without any purification. All UV-VIS spectra of PANi- (Emeraldine salt and Emeraldine base) were measured in water solution by adding one drop of original well dispersing sample to cuvette filled with distilled water.
Electronic absorption spectra of the well dispersed PANi-2 in water is shown in Figure 3. The original PANi –dispersion was evaporated on a watch glass in drying oven for 1 hr at 100 o C. The broad band of 1.0 wt% aqueous solution at about 350 nm to 420 nm represents electronic transition (p®p*) of the benzoid ring. The band at about 800 nm is due to quinoid ring [11].
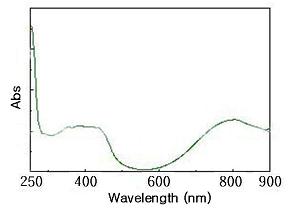
Figure 3. UV-Vis spectrum of TSA and HBr doped polyaniline salt (film casting).
UV-VIS spectrum of PANi-4 is shown in Fig.4. The broad peak at 785 nm (Emeraldine Salt) can be assigned to localized polaron structure. When emeraldine salt was dedoped by concentrated ammonia solution, the peak at 331 nm and 575 nm were appeared. These peaks are due to p®p* transition of benzoid ring [13] and benzoid-quinoid structure respectively. The peak at latter (575 nm) differs with different dedoping agent [14]. UV-VIS spectra of PANi-5 were also taken but there were no characteristic differences.
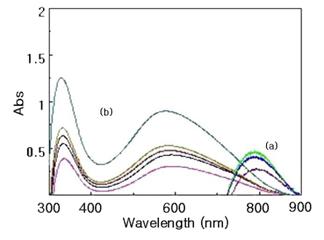
Figure 4. UV-Vis spectra of H2SO4-doped and NH4OH dedoped-polyaniline:
a) The intensity of peak of PANi (salt) is increasing with oxidation time (5 mins, 2 hrs, 3 hrs from bottom to top),
b) The intensity of peak of PANi (EB) is increasing with dedoping time (1 min, 30 min, 4 hrs, 5 hrs, 24 hrs; from bottom to top ).
3.4. FT-IR spectroscopy
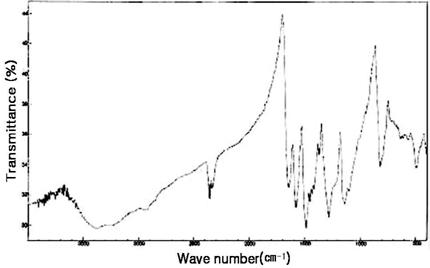
The FT-IR spectra of H2SO4-doped PANi/PVP composites (EB) with and without VA-044 are shown in Figure 5 and Figure 6, respectively. The broad band around 3450 cm -1 in both cases represent H-bonded NH stretching vibrations which generally appears in FTIR spectrum of PANi [15]. The peaks at 1582.6 cm -1 (quinoid ring), 1493 cm -1 (-C=C- of benzoid ring), 1280.7 cm -1 (B-C-N stretching mode), 1153.4cm -1 (Q=NH-B), are close resemblance with data published in literatures[13, 15]. The characteristic peak of carbonyl group (>C=O) of PVP could not be observed in the spectra. This peak seems to be merged with broad peak at 1582 cm -1 [13]. The peak at 2357 cm -1 is unknown peak that may be from –CºN of unknown byproducts.
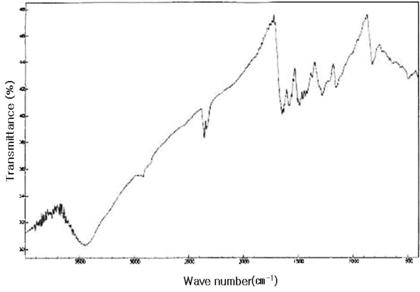
Figure 5. FTIR Spectra of PANi/PVP/VA-044 composite doped by H2SO4 and dedoped by NH4OH.
3.5. Scanning Electron Microscopy
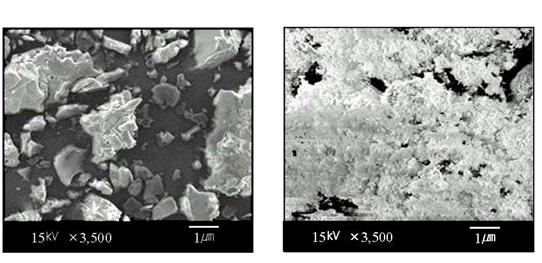
SEM photographs of two different PANi/PVP composites without and with VA-044 are shown in Figure 7. No separate domains for conducting and insulating components are visible in Figure 7(b), which indicates the closer association of components and homogeneity of sample [16]. Micrograph (b) appears to be much finer in size and homogeneity. The average particles sizes of PANi/PVP/VA-044 composite (£1mm) are smaller than PANi/PVP composite (~15 mm). The smaller sized particles seem to be responsible for the formation of conducting network.
(a) (b)
Figure 7. SEM photographs of PANi(EB)/PVP composites: (a) without VA-044, (b) with VA-044.
4. CONCLUSION
The PANi composites have been synthesized in aqueous acid medium. The characterization of PANi composites by using UV-VIS, FTIR spectrophotometer shows the formation of polyaniline. The dispersion and surface resistivity of different samples of Polyaniline synthesized in one acid or mixture of inorganic and organic acid using PVP and azo-initiator are higher than PANi without VA-044. The well dispersion of PANi only with azo-initiator indicates that it must have done some role. It reveals that azo-initiator plays a multiple-role: dispersion agent by forming H-bonds, initiator and controller for polymerization.
REFERENCES
1. Xing Rong Zeng and Tze-Man Ko. Polymer 39 , 1187 (1998)
2. I. Kulsxewicz-Bazar, M. Zzagorska, A. Bany, L.Kwiatkowski. Synthetic metal , 102 , 1385 (1999)
3. C.Weidlich, K.-M. Mangold and K.Juttner, Electrochmical Ac ta , 47, 741 (2001)
4. A.J. Epstein, J.Joo, C.-Y.Wu, A.Benatar, C.F. Faisst, Jr; J. Zegarski and A.G.MacDiarmid, Intrinsically conducting polymer: An Emerging Technology, M.Aldissi, ed.(Kluwar Academic pubs, Natherlands), 165 (1993)
5. J. Y. Lee, D.Y. Kim, C.Y. Kim , Synthetic Metal , 74, 103 (1995)
6. J. Y. Lee, K.T. Song, S.Y. Kim, Y.C. Kim, D.Y. Kim, C.Y. Kim, Synthetic Metal , 84, 137 (1997)
7. Y. Cao, P. Smith, A.J. Heeger, Synthetic Metal , 48, 91(1992)
8. Gue-Wuu Hwang, Kuan-Ying Wu, Mu-Yi Hua, Hsun-Tsing Lee, Shw-An Chen, Synthetic Metal, 92, 39 (1998)
9. E. S. Matveeva, R. Dlaz Calleja, and V. P. Parkhutil, Electrochemica Acta , 41, 1365 (1996)
10. Dennis E. Tallman, Youngun Pae, Guoliang Chen, Gorden P. Wierwagen, Brent Reems and V.J. Gelling, Conductive Polymer and Plastic in Industrial Aplication, page 201 (1999)
11. L. H. Huo, L. X. Cao, D. H. Wang, H. H. Cui, G.F. Zeng, S.Q. Xi, Thin Solid Film , 350 , 5 (1999)
12. Premamoy Ghosh, Samir K. Siddhanta, S. Rejaul, Amit Chakrabarti, Synth.Met. 123 (2001) 83
13. A. B. Samui, A. S. Patankar, R. S. Satpute, P. C. Deb, Synth. Met., 125 , 423 (2002)
14. Hong-Quan Xie, Yong-Mei Ma, Jun-Shi Guo, Synt.Met ., 123 , 47 (2001)
15. Dan Shan, Shaolin Mu, Synth. Met ., 126, 225 (2002)
16. Rupali Gangopadhyay, Amitabha De, Goutam Ghosh, Synth. Met ., 123 , 21 (2001)
[ BWW Society Home Page ]
© 2002 The BWW Society/The Institute for the Advancement of Positive Global Solutions