The Sciences: Chemistry:
Enhanced Photocatalytic Activity for Aromatic Organics Using Photoelectrocatalytic System (
By Dr. Jin-Wook Ha, Hak-Soo Kim*,
and Chul-Hee Han**
Department
of Energy and Environmental
*Division of Materials
and Chemical Engineering,
**Department of Chemistry,
ABSTRACT
The photoelectrocatlytic
system is a discharge photocatalytic system aimed at
enhancing the photocatalytic performance. In this
study, we have obtained experimental data revealing the basic mechanism of the photoelectrocatalytic system. We also report removal of xylene isomers in air using the photoelectrocatalytic
system, and these data are compared with our previous data on benzene and
toluene. Our analysis based on the Lagmuir-Hinshelwood(L-H) kinetic
model shows that the rates of degradation are in the order; benzene <
toluene < xylene, in the photoelectrocatalytic
system, and explanations are given for the trend.
1.
INTRODUCTION
TiO2 photocatalysts
show wide range of photocatalytic activities
including decomposition of aliphatic and aromatic compounds and killing of
bacteria both in air and aqueous environment under ambient condition[1-3].
In addition to high photocatalytic activities of wide
range, photochemical stability and low cost for manufacture have given TiO2
photocatalysis good prospects for clean technology
against environmental pollutions[4-5]. However, wide
spread commercial use of TiO2 photocatalysts
has been slow, mainly due to the large band gap energy(3.2eV)
and rapid recombination of photogenerated electrons
with holes[1].
Our approach to these problems was to
retard rapid recombination between the photogenerated
electrons and holes in TiO2, and we accomplished this by applying high voltage
across a layer of TiO2, and it is this feature that distinguishes photoelectrocatalytic system from conventional photocatalytic systems. Our photoelectrocatalytic
system is based on the idea that the photogenerated electrons
in a layer of TiO2 would move toward a cathode with application of
high voltage across the TiO2 coated Aluminum plate. In our system,
aluminum plate is used as a substrate for TiO2 and also serves as a
cathode. According to our scheme, moving photogenerated
electrons toward a cathode would have the same effect as moving these electrons
away from the holes, which would have the effect of retarding recombination of photogenerated electrons with holes. Our recent experiments
on benzene and toluene showed higher rates of removal with high voltage on
compared to high voltage off, which supported our scheme partially[6].
In this study, we have extended the application of the photoelectrocatalytic
system to the removal of xylene isomers. With an
inclusion of xylene, our list of aromatic compounds
includes benzene, toluene, and xylene, in the order
of increasing methyl group. Moreover, all three types of xylene
isomers were chosen to investigate possible effect of substituent
position on the rate of degradation in our photoelectrocatalytic
system. Our continuing effort to provide direct evidence to support our scheme
has resulted in the measurement of current due to flow of the photogenerated electrons in TiO2 toward aluminum
plate. Hereby, we report for the first time to our knowledge a measurement of
current from TiO2.
2.
EXPERIMENTAL
The detailed
description of our photoelectrocatalytic system was given in our recent publication[6]. The reaction was carried
out in a batch type glass vessel at 1 atm and ambient
temperature, and the photoelectrocatalytic system
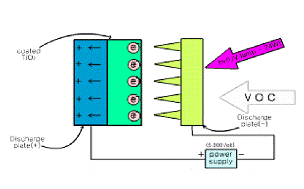
along with our scheme is shown in Fig 1. Air
flowed through a mass flow controller into the saturator placed in a constant
temperature bath at 20°C and 1atm, and was mixed with 90 % air coming from another
mass flow controller in order to obtain predetermined concentrations(500ppm,
2,000ppm, 10,000ppm) of aromatic compound in air. This aromatic mixture flowed
into and out of a 10l batch reactor
initially to allow adsorption to take place in the reactor. Samples of outlet
mixture were periodically analyzed with a gas chromatograph(GC, HP6890)
equipped with a flame ionization detector and a capillary column(HP 5) to be
compared with the target composition. When the target composition was achieved,
the inlet and outlet of the reactor were closed, and photoelectrocatalytic
degradation of the aromatic compound was carried out in the reactor. During the
experiment gas samples were taken at 30 min. interval for GC analysis to
monitor variations in the concentration of the aromatic compound.
The photocatalyst
used in this study was sol type titanium dioxide(YJPC,
For the current measurement, aluminum
plate coated with TiO2 was used as a cathode while an array of Cu
strips in the shape of saw tooth was used as an anode, and a digital
multi-meter(HP 344501A) was connected to aluminum plate and the power supply as
shown in Fig 1(b).
(a)
(b)
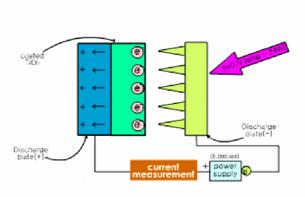
Fig. 1. The schematic diagram of using systems;
(a) photoelectrocatlytic system and (b) current measurement system.
The current measurement was performed
with the application of 5,000 volts DC bias in three stages; (1) no UV
irradiation for 1 min., (2) UV irradiation for 1 min., and (3) no UV
irradiation for 1 min. These stages were repeated many times for signal
averaging due to the nature of minute signal level, and aluminum plates with
and without TiO2 coating were used in this experiment for comparison
purposes.
3. RESULTS AND
DISCUSSION
The current measurement data are shown
graphically in Fig. 2. Fig. 2(a) was obtained from the experiment with TiO2
coated aluminum plate, whereas Fig. 2(b) was from aluminum plate without TiO2
coating.
(a) (b)
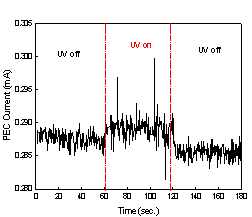
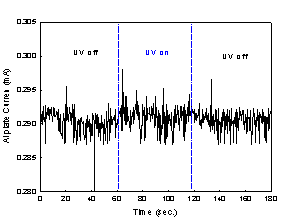
Fig. 2. The current
measurements with metal plates;
(a) TiO2
coated Al plate and (b) uncoated Al plate.
The current levels in Fig. 2(b) stayed
virtually constant over three stages of our experiment, and they correspond to
a bias current. On the other hand, a jump of current level at the onset of the
second stage and a drop at the onset of the third stage were observed in Fig.
2(a). In addition, the current level in the second stage stayed clearly above a
bias current. The lower bias current in Fig 2(a) compared to that in Fig 2(b)
can be attributed to higher electrical resistance of TiO2 coated
aluminum plate. Thus, both TiO2 coated aluminum plate and UV
irradiation were necessary for the increase in current, and this in turn shows
that the increase in current is due to the flow of photogenerated
electrons from TiO2 toward aluminum plate substrate.
As to the photocatalytic
removal of xylene in air, the initial removal rates
for three xylene isomers(Fig.
3) were analyzed according to Langmuir-Hinshelwood(L-H)
kinetic model,
![]() (1)
(1)
where k is the surface rate constant, Kapp
is the apparent adsorption coefficient, and Ceq
is the reactant equilibrium concentration.
(a) (b) (c)
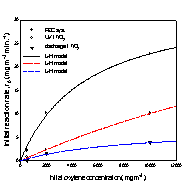
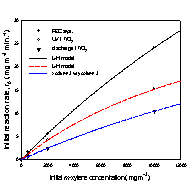
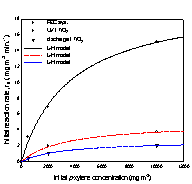
Fig. 3. Initial removal rate of
xylene isomers versus initial concentration;
(a) o-xylene, (b) m-xylene, and (c) p-xylene.
The kinetic parameters k and K were
obtained using linear least squares analysis, and the values for k are
presented in Table 1. In Table 1, “PEC sys” refers to the photoelectrocatalytic
system, “UV/TiO2” refers to the conventional photocatalytic
system, and “discharge/TiO2” refers to the system under plasma
effect.
The removal rates of the aromatic
compounds in Table 1 are in the order; benzene < toluene < (o-, p-)xylene, and this order correlates
with increasing electron-donating substituent to the
benzene ring. Another order common to all three systems is; m-xylene < o-xylene < p-xylene. Between m-xylene and
toluene, the removal rate of m-xylene is higher in
the photoelectrocatalytic system, but lower both in
the photocatalytic system and discharge system. For xylene isomers most pronounced increase in the removal
rates occured going to the photoelectrocatalytic
system from other systems, and it is in the photoelectrocatalytic
system that all three xylene isomers show higher
removal rates than toluene.
Table 1. Langmuir-Hinshelwood(L-H) parameters(k)
obtained in the photocatalytic degradation of
aromatic compounds.
(k: ml m-3 min.-1)
|
Compounds |
PEC
system* |
UV/TiO2 |
Discharge/TiO2 |
|
Benzene |
3.07 |
2.16 |
0.97 |
|
Toluene |
7.13 |
6.19 |
2.96 |
|
m-xylene |
15.50 |
3.58 |
2.52 |
|
o-xylene |
33.67 |
7.75 |
6.02 |
|
p-xylene |
48.78 |
11.88 |
7.59 |
*PEC system: Photoelectrocataytic system
The
higher removal rates for all the aromatic compounds in the photoelectrocatalytic
system are attributed to the reduced rate of electron-hole recombination in TiO2,
which is supported by our current measurement experiment.
4. CONCLUSIONS
The degradation of benzene, toluene, xylene isomers was carried out in
the discharge potoelectrocatalytic system consisting
of TiO2 thin film, its aluminum substrate as cathode, anode of Cu
strips, high voltage power supply and UV lamps. Compared to either the plasma
effect only or the photocatalytic effect only, higher
degradation rates were observed for benzene, toluene, (o-, m-, p-)xylene in the discharge photoelectrocatalytic
system. The higher rates of degradation for these compounds in the photoelectrocatalytic system are attributed to the longer
lifetime of holes in TiO2 due to reduced electron-hole recombination,
which is supported by the detection of current flow from the photoexcited TiO2 only in the photoelectrocatalytic system. Thus, compared to the systems
of either the plasma effect only or the photocatalytic
effect only, our photoelectrocatalytic system would
be least limited by the number of holes available in TiO2 to the
radical reaction. Accordingly, the degradation rates in the order; benzene <
toluene < (o-, m-, p-)xylene,
in the photoelectrocatalytic system can be correlated
with the increasing number of electron-donating substituent
to the benzene ring. Furthermore, this suggests that the reaction determining
the overall degradation rate favors strong electron donor molecules. Finally,
different rates of degradation among three xylene
isomers show the effect of substituent position, and
the nature of such an effect will be discussed in our future publication.
Acknowledgements
This work was supported by
REFERENCES
1.
M.R. Hoffmann, S.T. Martin, W. Choi, and D.W. Bahnemann, Chem. Rev., Vol. 95, 69(1995).
2.
K. Sunada, Y. Kikuchi, K. Hashimoto, and A. Fujishima, Environ. Sci. Technol.,
Vol. 32, 726(1998).
3. J.C. Ireland, P. Klostermann, E.W. Rice, and R.M. Clark, Appl.
Environ. Microbiol., Vol. 59,
1668(1993).
4. A.L. Dibble and G.B. Raupp, Catal. Lett., Vol. 4, 345(1990).
5.
A. Mills, R.H. Davies, and D. Worsley, Chem. Soc.
Rev., Vol. 93, 417(1993).
6.
H.K. Kim, E.A. Lee, J.H. Lee, C.H. Han, J.W. Ha, and Y.G. Shul,
Intern. J. Photoenergy, Vol. 5, No. 1,
3(2003).
[ BWW Society Home Page ]
© 2007 The Bibliotheque: World Wide Society