Chlorine
Chemistry for
Starting
in the 1940s
With
Regard to the Inventors and Their "Fights"
by Prof Dr Randolph Riemschneider
and
Central Institute of Chemistry, Universidade Federal de
In the 40s and 50s, particularly in the Second World War and the Postwar Era, chemical pest control agents based on chlorine compounds of the DDT- and DIENE-groups as well as Gammexan as so-called "halocarbon class contact insecticides" (1, 2) played an important role. Thousands and thousands of publications appeared in the frame of chemical pest control research; in the fight against the “superpower insect” many harvests could be saved, illnesses like malaria be successfully contained. There were internal fights and altercations with respect to copyrights and Nobel Prize(s) related to DDT and DIENE group insecticides, latter based in hexachlorocyclopentadiene chemistry. Consideration is also given to the early references to the calculation of the use and risk in the widespread application of chemical pest control agents. -- The editor.
At the start of the 1870s the doctoral candidate O Zeidler in Stasbourg (3) synthesized - in the frame of investigations into the BAYER condensation - from 2 mol chlorobenzene and 1 mol chloral the compound C14H9Cl5, loosely known as dichlorodiphenyltrichloroethane (I): Fig 1. Some 70 years later the compound became interesting again when Dr P Müller - supervised by Dr P Läuger - again produced C14H9Cl5 (I) in the company labs of GEIGY AG, Basle while searching for new, effective stomach poisons. GEIGY patented I and analogues as general insecticides at the start of the 40s and brought preparations based on I onto the market under the name "Gesarol" for protecting crops and "Neozid" for combatting ectoparasites. But only the Americans recognized the actual value of I in the second world war. They coined the term DDT, derived from dichlorodiphenyltrichloroethane (Fig 1).
Cl3C-CHO + 2 C6H5Cl → C14H9Cl5
(I)
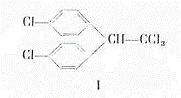
Fig 1: Synthesis of dichlorodiphenyltrichloroethane (I) from 1 mol chloral and 2 mol chlorobenzene [BAYER condensation] (6)
Examples of successes with DDT in WW2: the typhus epidemic, transmitted by lice, was successfully combatted in Naples in 1943; the dangers from insects (eg malaria) were considerably reduced on the Asian fronts by spraying the insects with DDT before troop landings; during and after the second world war harvests were saved by means of DDT and thus the food for many people assured.; malaria was contained worldwide with the aid of DDT and its analogues (above all DDD) and thus the lives of many people[1]. Reasons enough to initially play down the concerns necessarily related to the widespread use of chemical toxins, when balancing the use against the risk (4a-4d).
By reason of the successes mentioned, the Nobel Prize Chemistry
Committee in
Strange things happened at GEIGY's some time before the prize was awarded. The directors Mylius and Koechlin (5) published a paper in 1946 which they stated the data on "DDT" published by P Läuger, H Martin and P Müller in 1944 needed correcting in part and that the latter author had known nothing of these inaccuracies. And so only Dr Paul Müller was awarded the Nobel Prize, head of research Dr Läuger and Dr Martin were ignored.
After the destruction of RUHRÖL GmbH's hydrogenation works, the author lived in Thuringia (Erfurt, Jena) from 22 July 44 till the end of 1946 and considers himself lucky not to have known (due to the circumstances at the time) about the misleading data in the publication by Läuger, Martin and Müller before mid-1946; due to the chaos of war specialist journals were then largely unavailable in Germany, American troops entered Erfurt on 12 Apr 45, being replaced by the Soviet occupying power some months later. The postal service ground to a halt, there was no possibility of foreign contacts in mid-1946. Those data might well have negatively influenced the author's plans with respect to "halocarbon-based contact insecticides" and possibly even have prevented important corrective experiments. At a time when dichlorodiphenyltrichloroethane (I) was not yet famous as DDT, the author had already synthesized a large number of DDT analogues (6) as well as other halocarbons (7b-9d) and thus laid the foundations for a new group of contact insecticides, viz the DIENE group (2, 11, 16).
The author had already stumbled across the extraordinarily high and unexpected reaction of the DIENE C5Cl6
with unsaturated compounds when he prepared it for the first time at the German
Army Explosives and Warfare Agents Research Establishment in Prague in 1943
(8a, 8b), where he synthesized C5Cl6 through hypochlorite
to C5H6 in Feb 43 to study its properties and compare
them with the "explosive" dichloroacetylene (C2Cl2).
While working in RUHRÖL's hydrogenation works from 01 Apr 43, the author
(parallel to his actual job) used C5Cl6 as the starting
material for synthesizing "halo compounds with a new spatial distribution
of chlorine" (2), this in the frame of the task that he has set himself
"Constitution (configuration) and action of of halocarbon-based contact
insecticides". This work led to the discovery of a new group of
insecticides, namely the DIENE group, that was a thousand times more potent
than DDT. M 410 (7b-9d) was later on the market in
Contrary to all expectations, C5Cl6 reacted - despite of its high substitution - with unsaturated compounds of all kinds, sometimes exothermic (8). In collaboration with Farbwerke HOECHST this work later led to the insecticide and commercial product Thiodan (10a, 10b). It might be referred here to (16) for further information.
The author wrote about the "development of DIENE group
insecticides in
The main objective of the article published in an uninvolved third
country (11) was to show what - as far as the author was aware at the time -
had taken place with respect to the discovery and development of the DIENE
group insecticides in
1) It was pointed out in the appendix to (11) from 1951 that the
patent dispute between HYMAN Co of
2) Dr Hyman had turned to the author, the German inventor of the
DIENE group insecticides, to get help against VELSICOL (9d), namely through
proof that the author had made the invention concerned before VELSICOL.
The circumstances in
3) In the Swiss patent applications (12) pest control agents were
claimed containg chlorinated
hydrocarbons, prepared through chlorination of cyclopentadiene with alkaline
hypochlorite solution and subsequent direct chlorination of the reaction
mixture's organic phase, preferably postchlorinated to Cl8 or Cl7,
related to C10. On the basis of this application, Farbwerke HOECHST
applied - with the author's agreement - for patent F 8439 Iva/45 1) entitled
"
4) The hypochlorite method dealt with in the patent application described at (8) was developed in 1947-49 by the author and co-workers ready for production, delivering a good yield of C5Cl6. [In agreement with Farbwerke HOECHST the author however only classified this outstanding hypochlorite method as a lab method in the publication (11)]. By means of this method Farbwerke HOECHST manufactured C5Cl6 on a large scale from 1951, eg thiodan synthesis (10a, 10b).
5) The inventors named, Hyman and Riemschneider, were once again the topic of conversation, namely in the Nobel Prize committee for chemistry in 1973: in 1972 two topics from the cyclopentadiene field were discussed as being worthy of a Nobel Prize:
I cyclopentadiene metal complexes: sandwich compounds
II DIENE synthesis with superchlorinated DIENES and uses: hexachloro- cyclopentadiene chemistry
The author heard about it when two American colleagues visited him
in
The Nobel Prize for I was awarded to Profs E O Fischer (
6) As shown above, there are officially two inventors of the
DIENE group insecticides, more precisely of the processing of the extraordinary
reactivity of hexachlorocyclopentadiene, a discovery that triggered thousands
of follow-up papers throughout the world. In the opinion of Farbwerke HOECHST's
management, in particular of Dr Scherer of the plant protection dept, however it
all goes back to the author: Immediately after American troops conquered
and occupied
Contact insecticide research, like antibiotics research (Fleming inter alia), was "fashionable" in the 40s and 50s. Sulphonamides (DOMAGK) were superseded by antibiotics, these in their turn by vitamins. The talk at the time was of the vitamin fuss. "Even science has its fashions!"
From the mid-60s on there was a move away from chemically based pest control agents and towards "damning" them, but forgetting what they had achieved. The author had already expressed his critical views on pest control by the widespread use of chemicals in a series of lectures (refs: 4a-d, 13a,b) 1948-51, ie calculating the risk and benefit of using insecticides. The manuscripts of all these lectures - together with other documents (seperata) - were sent to the environment protection writer Rachel Carson at her request (secretary: Elisabeth Schölzel). These comprehensive texts were, unfortunately, not quoted by Ms Carson in her book "Silent Spring", published in 1962, although they contained fundamentals and in any event "fertilized" the book. The author refrained from legal steps in view of Ms Carson's poor state of health.
The following example shows how valued the help given by an
insecticide is in

Plate
2: Monument for E 605 in
The author wishes to thank BAYER's Kekulé Library for permission to use the photo.
Bibliography:
(1) R Riemschneider
Coining the term "Halocarbon-based
contact insecticides" (Prägung des Begriffes“Kontakt-Insektizide
auf Halogenkohlenwasserstoffbasis“),
June 43 - planning, 12 lab reports from May - July 43
Works lab of RUHRÖL GmbH (parallel to actual job, making use of staff freed up by bomb damage), continuing synthesis and collecting of halo compounds in lab of Inst of Hyg at Univ of Jena from Oct 44 till Mar 45 and in Inst of Pharma at Univ of Jena from Sept 45 till end of 46. Above all seeking compounds with a new spatial distribution of chlorine (2, 7a)
(2)
R Riemschneider (lecturer)
2 lectures
Lecture 1: "The
spatial distribution of Gesarol's active ingredient (later DDT) and of
halogenated adducts from hexachlorocyclopentadiene and cyclopentadiene: DIENE
group insecticides, eg M 410 (C10H6Cl8) as
well as M 393, M 377, M 344"(„Über
die Raumverteilung der Chloratome des Gesarol-Wirkstoffes (später DDT) und der
halogenierten Addukte aus Hexachlorcyclopentadien und Cyclopentadien:
Insektizide der DIEN-Gruppe, z. B. M410 (C10H6Cl8)
sowie M393, M377, M344.“)
20 min lecture in Inst of Hyg at Univ of Jena on 15 Dec 44, invited by Prof Dr H Schloßberger (chair). Present: Profs Dr H Bredereck (org chem), Dr H Britzinger (tech chem) [at instigation of Dr G R Schultze, Braunschweig Tech Coll, author's doctoral supervisor], Dr F Hein (anorg chem), Dr H Keller (phar-ma) and Dr Scheffler, dean of Fac of Math and Nat Sc
Lecture 2: "The naturally occurring insect toxin cantharidin (II) as a model substance for developing synthetic insecticides with a five-membered ring structure, eg M 410 (III)" („Das natürlich vorkommende Insektengift Kantharidin (II) als Modellsubstanz für die Entwicklung synthetischer Insektizide mit Fünfring-Struktur, z. B. M 410 (III)“), given in colloquium of Inst of Hyg, Univ of Jena on 10 Dec 44, chair Prof Dr H Schloßberger
Plate 1: Cantharidin (II) as model substance for insecticides with five-membered ring structure, eg M 410 (III)
II
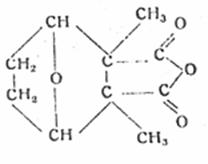

III
(3)
O Zeidler
Doctoral thesis 1872, Strasbourg, quoted in:
Ber dtsch chem Ges 7 180 (1874)
(4a) R Riemschneider
Two-part lecture given in chem colloquium of BUNA Werke, Schkopau on 29 Jan 48
Part 1: "Contact insecticides of DDT, HCH and DIENE groups"(„Kontakt-Insektizide der DDT-, HCH-, DIEN- Gruppe“)
Part 2: "Critical comments on chemical pest
control and alternatives"(„Kritische
Anmerkungen zum Thema der Chemischen
Schädlingsbekämpfung und Alternativen“)
Summary of part 1 in Pharmaz Zentralhalle 87 132 - 134 (1949), rev C 1949 II, 1226 (Verlag Chemie)
Part 2: Mitt physiolog chem Inst
(4b) R Riemschneider
Two-part lecture given in chem colloquium of LEUNA, Merseburg on 11 Feb 48 and in Werk KAUSTIK, Wolfen on 14 Mar 48
Part 1: "Halocarbon-based contact insecticides and phosphorous-bearing insecticides, particularly so-called 'hexaethyltetraphosphate' (HETP)"( „Über Kontakt-Insektizide auf Halogen-Kohlenwasserstoffklasse und über phosphorhaltige Insektizide, speziell über das sogenannte «Hexaethyl- tetraphosphat»“)
Part 2: "Criticism of large-scale pest control
with chemical toxins and alternatives"(„Kritik zur großflächigen Schädlingsbekämpfung mit chemischen
Giften und Alternativen“)
The author was invited to give the lecture in two parts by the managements of the BUNA, LEUNA and KAUSTIK works, who wanted both the latest infomation on "CHEMICAL PEST CONTROL RESEARCH" and a critical appraisal thereof. Chief engineer Richter of the LEUNA Werke had informed the author in confidence that LEUNA and BUNA were faced with deciding whether or not - and if so, to what extent - they should become involved in the field of chlorine chemistry. The tendency was "not".
(4c) R Riemschneider (lecturer), W Cohnen
Lecture "Dangers and precautionary measures when using modern insecticides"(„Gefahren und Vorsichtsmaßnahmen bei der Anwendung neuzeitlicher Insektizide“)
given in colloquium of Inst of Physiolog at Humboldt
Univ of
Published in Arzt und Patient 63 vol 3 1950. Pp 1 and 2 by Dr W Cohnen, using Riemschneider's recorded lecture notes - with his permission. Ms given to press in June 49. The lecture contained early and emphatic warning of the toxic effects of halocarbons on warm- and cold-blooded animals.
The author referred to other methods of pest control envisaged and in part taken in hand, entitled: "Less chemical pest control" in (14) at PROJ VI 4
(4d) R Riemschneider
"Controling pests with chemical agents
- consequences for humans, animals and plants - effects of insecticides and
follow-up products on the food chain - resistance problems"(„Bekämpfung von Schädlingen mit chemischen Mitteln ---
Folgen für Mensch, Tier und Pflanzen ---
Eingriff von Insektiziden bzw.
Folgeprodukten in die Nahrungskette ---
Resistenz-Probleme“.)
Lecture given in round-table talks at afternoon tea held regularly in Inst of Math at Free Univ of Berlin on Thursdays during term on 16 Nov 50. Invited by chair: Prof Dr A Dinghas, institute director.
Present: Profs Dr M Fischer (physiolog chem), Dr F Herter (zoo), Dr W Heubner (pharma), Dr Jakobson (maths, visiting prof from Norway), Dr W Lautsch (org chem), Dr F Richter (geology), Dr G Schenck (pharm chem), Dr F Scherer (Plant Protection Dept, Farbwerke HOECHST, Frankfurt-am-Main), by special invitation Dr Ivan Stranky (physic chem).
Ms Oct 50, 32 p (unpublished)
(5) A Mylius, H Koechlin
Correction of work by P Länger, H Martin, P Müller [Helv cim Acta 27 892 - 928 (1944)] "The constitution and toxic action of natural and new synthetic insecticidal substances" („Über die Konsititution und toxische Wirkung von natürlichen und neuen synthetischen insektentötenden Stoffen“)Helv chim Acta 29 405 - 411 (1946), rev in Chem Zbl 1947 II 30 (Verlag Chemie)
(6) R Riemschneider
Extracting the active ingredient
of the (Colorado) potato beetle control agent GESAROL and identifying the
lipoid-soluble constituent as C14H9Cl5 - with
the aid of GEIGY patents reviewed in Chem Zentralblatt
[C 1942 II 706, ref French patent 870689 of 07 Mar 41] - more precisely as the
condensation product from chloral and chlorobenzene (Extraktion des Wirkstoffes des Kartoffelkäferbekämpfungs-mittels
GESAROL und Identifzierung des lipoidlöslichen Anteils als C14H9Cl5
- unter Zuhilfenahme von GEIGY-Patenten, die im Chem. Zentralblatt [C 1942, II, 706 betr. franz. Pat. 870689 vom 7.3.1941] referiert
waren – genauer als Kondensationsprodukt aus Chloral und Chlorbenzol,)
Lab report May 43, 8p. Experiments conducted in works
lab of Ruhröl GmbH,
Preparing
β,β,β-trichloro-α,α-bis-[4-chloro-phenyl]-ethane [dichloro-diphenyl-trichloroethane] from 1 mol chloral and 2 mol chlorobenzene C14H9Cl5
to prove the structure of the isolated GESAROL active ingredient
Lab report June 43, 6 p. Experiments conducted in
works lab of Ruhröl GmbH,
The talk here is of the insecticide known by the
abbreviation DDT, derived from the chemical designation dichlorodiphenyltrichloroethane,
made world-famous by the Americans in the second world war - but that only
became widely known in
(7a) R.
Riemschneider
On
using hexachlorocyclopentadiene as starting material for “synthesis of chlorine
compounds with new spatial distribution of chlorine”: OET group
(later: DIENE group) (Über den Einsatz von Hexachlorcyclopentadien
als Ausgangsmaterial zur „Synthese von Chlorverbindungen mit neuer Verteilung
von Chlor im Raum“: OET-Gruppe
(später DIEN-Gruppe))
Lab reports, 9 p, Dec 43 and May 44.
Experiments conducted at RUHRÖL GmbH,
“A new type of insecticidal halogen compounds,
starting from C5Cl6 – after first tests, very promising:
M 410” (Ein neuer Typ insektizider
Halogenverbindungen, ausgehend von C5Cl6 - nach ersten Testversuchen vielversprechend:
M 410.)
Lecture given in Inst of Pharmaceutics, Univ of Jena on 31 Jan 45
Insecticidal activity of M 410 on Calandra granaria & Blatta orientalis greater than DDT
(7c) R Riemschneider
On the remarkable properties of post-chlorinated
adducts from hexachloro-cyclopentadiene and cyclopentadiene, C10H6Cl6
– M 410 identified as octa-chloroendomethylenetetrahydrohydrindene (OET), C10H6Cl8
(mol wt 410) (Über die bemerkenswerten Eigenschaften
nachchlorierter Addukte aus Hexachlorcyclopentadien und Cyclopentadien, C10H6Cl6.
– M 410 identifiziert als Oktachlorendomethylen-tetrahydrohydrinden (OET), C10H6Cl8
(Molgewicht 410))
![]() C10H6Cl6 + Cl2 C10H6Cl8
C10H6Cl6 + Cl2 C10H6Cl8
Lab
reports 1944 and to Mar 45. Experiments conducted at RUHRÖL GmbH and in Inst of
Hyg, Univ of Jena
Hexachlorocylopentadiene, C5Cl6: 2 lectures
1: “The action of sodium hypochlorite on
cylopentadiene: hexachloropenta-diene and higher-melting reaction products” (Über
die Einwirkung von Natriumhypochlorit auf Cyclopentadien: Hexachlorpentadien
und höher schmelzende Reaktionsprodukte)
Lecture given on 15 Feb 43 (20 p)
2: “Properties and reactions of C5Cl6”
(Eigenschaften und Reaktionen des C5Cl6)
Both lectures given in German Army Explosives and
Warfare Agents Research Establishment in
The “example” of dichloroacetylene, C2Cl2,
made it seem appropriate to test if C5Cl6 was also suited
for an explosive or warfare agent. Result: negative
Lab reports, 30 p,
RUHRÖL GmbH,
(9a) R Riemschneider
"A new type of organic halo compounds:
hexahalocyclopentadiene adducts - testing the adducts and adduct derivatives
for insecticidal activity on Calandra granaria, Blatta orientalis, Musca domestica"(„Über einen neuen Typ organischer Halogenverbindungen: Hexahalocyclopentadien-Addukte
– Prüfung der Addukte und
Adduktderivate auf insektizide Wirksamkeit an Calandra gra naria, Blatta orientalis, Musca
domestica“)
Lecture given in the framework of doctoral proceedings in colloquium of Inst of Technology, Univ of Jena on 10 Feb 45, chair: Prof Dr H Brintzinger
This and previous lecture (8b) were very early on official, detailed bulletins on the OET (DIENE) group insecticides; cf (9c)
(9b) R Riemschneider
"DDT and M 410 - halocarbon-class contact insecticides" ( „DDT und M 410 – Kontakt-Insektizide der Halogenkohlenwasserstoffklasse“)Published in the daily newspaper "Thüringer Volk", spring 1946: PROJ VI, Pl 2 in (14) written at the instigation of O Trillitsch, the editor, in an easily understandable way, with reference to the discoverer of DDT's active ingredient, Dr Paul Müller of Wädenswil, Switzerland (employed at Geigy in Basle under the scientific supervision of Dr P Läuger) and with reference to a new development in the field of insecticide chemistry: M 410 (1943/44)
Cf the article in "Thüringer Volk" by editor O Trillitsch entitled: " M 410 and M 344, rival for DDT?" (14)
(9c) R
Riemschneider, A Kühnl
New halocarbon-class contact insecticides: M 410, M 377, M 344, and others: f.i. M 414 (Neue Kontakt-Insektizide der Halogenkohlenwasserstoffklasse: M 410, M 377, M 344, und andere: z.B. M414.)
Bulletin of Inst of Physiolog Chem, Univ of
(9d) R Riemschneider
"M 410 - Preparation and properties"(„M 410 --- Preparation and properties“)
Lecture given in small circle of chemists from Hyman Co, Denver, Colorado, USA in June 49 - lecture ms of 15 May 49, 30 p (unpublished); cf (14), there Pl 1 PROJ XI
The lecture resulted from an invitation by Julius Hyman, the American inventor of chlordan:
Dr Hyman had legal problems with his former
employers, Velsicol Corp of
(10a) R Riemschneider
Thiodan
and related compounds (Thiodan
und verwandte Verbindungen)
Bull I (with J C Hilscher): The quantative determination of thiodan
Z analyt Chemie 165
278 - 280 (1959)
Bull II (with F Franco, R
Schlepergrell, B Götze, R Remke):
"The stereochemistry of norbornene series
cyclic sulphite esters and sulphoxides"
Botyu Kagaku,
Bull III (with W Ernst): butane-2-diol-1,4-cyclosulphite and other sulphite esters
Z Naturforschg 15b
552 - 554 (1960)
Bull IV (with J C Hilscher) The state of research into of thiodan isomerism
Z Naturforschg 15b
809 - 810 (1960); further Bulls in (16)
(10b) R
Riemschneider, R Sato
Selenious acid esters of
1,4,5,6,7,7-hexachlorobicyclo-(2,2,1)-heptene-(5)-bis-hydroxymethylene-(2,3) [Selenigsäureester des
1,4,5,6,7,7-Hexachlorbicyclo-(2,2,1)-hepten-(5)-bis-hydroxymethylens-(2,3)) vom
Schmp. 131°C (Selen-Analoges des Thiodans)from m p 131oC (selenium
analogue of thiodan)]
Ms 6 p Jan 59 (secreted at request of Farbwerke
HOECHST,
(11) R Riemschneider
Chimie et Industrie (Paris)
64 675 - 698 (1950)
Addendum: 65 60
(1951)
(12) R
Riemschneider (inventor), O Matter (applicant)
Two patent
applications entitled "
1)
Insecticides based on hexachlorocyclopentadiene (C5Cl6)
adducts from cyclo-pentadiene and hypochlorite and post-chlorination of
chlorine number 6 [or 51] to 7 or 9 [or 6 to 71]"
„Insektizide auf Basis von Hexachlorcyclopentadien-Addukten (C5Cl6-Addukten)
aus Cyclopentadien und Hypochlorit und Nachchlorierung von Chlorzahl 6
[bzw. 51)] auf 7 bis 9 [bzw. 6
bis 71)]“
1) If pentachlorocyclopentadiene forms as a by-product while preparing C5Cl6 by hypochlorite-chlorination
2) "Insecticides based on adducts from tetrachlorodifluorocyclopentadiene (C5Cl4F2) and cyclopentadiene and post-chlorination on chlorine number 6: C10H6Cl6F2"(„Insektizide auf Basis von Addukten aus Tetrachlordifluor-cyclopentadien (C5Cl4F2) und Cyclopentadien und Nachchlorierung auf Chlorzahl 6: C10H6Cl6F2“)
Due to the circumstances of the time, it was only
possible to apply for these patents in May and June 47 with the help of Swiss
friends, namely Dipl Ing O Matter [naming Dr R Riemschneider as inventor],
after the author had left the Soviet occupation zone for
by occupying powers] Only the general formulas A and B were used in application 1 for the insecticidal and / or fungicidal active agents of the pest control agents to be patented to
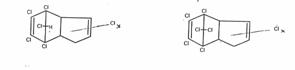
A B
avoid a collision with the structural formulas, already worked out and published, of the chlorine compounds C10H6Cl8 (M 410) [or C10H7Cl7] and the adduct precursors C10H6Cl6 [or C10H7Cl5], known to be active.
Patent claim to application 1:
Pest control agents, containing chorinated hydrocarbons of the general formulation A and B, that are obtained as follows: Chlorination of cyclopentadiene with alkaline hypochlorite solution [1 : 6 to 1 : 7], initially in the cold, then at temperatures up to 60oC and subsequent direct chlorination of the organic phase of the reaction mixture in the presence of UV light or other catalyzers, preferably post-chlorinated on a chlorine number of a reaction mixture from 8 or 7, related to C10. A patent application by Farbwerke HOECHST became known on 06 Sept 56 [F 8439 Iva/45 I, entitled Pest Control Agents, applied for on 26 Feb 52, ie 5 years after receipt of the text of the patent applications of 26 May and ** June 47 (12)] in which the active agents formulated by the author in the patent claim to application 1 were claimed by reason of tests by Hoechst's Plant Protection. The chemical page of the specification corresponds exactly to the application text (12) the author had made available to the management 5 years before the application. However, so as not to endanger HOECHST's application, the author agreed in 1955 that literature (12) as well as his papers on hypochlorination not be quoted after the fact. The author forwent being named as the inventor as the experimental part of the application by and large only contained biological data and as it was already clear at the time that - in view of developments in America: aldrin, dieldrin - the object of the patent would hardly become significant (16).
(13a) R Riemschneider
"
Lecture given in Inst of Math, Free Univ of Berlin on 22 Feb 51, chair: Prof Dr A Dinghas; cf (4d)
The lectures (4d, 13a) were repeated at colloquiums in Farbwerke HOECHST's Plant Protection Dept on 06 and 07 June 51 at the instigation of Dr F Scherer, director of the department, and at the invitation of the company's management. Publication of the lecture was deferred at the management's request so the scientists from the Plant Protection Dept could check and discuss the proposals made and would have time to study the literature. There was talk for the first time in the discussions of a "chemical cudgel", but also of not talking too much about it.
Neem tree products were dealt with in great detail in these lectures, 50% of the 01 Mar 51 lecture being devoted to them. Ms Jan 51, 30 p (unpublished)
(13b) R Riemschneider
Lecture given on 01 Mar 51, analogous to previous one (4d, 13a)
"Pest control avoiding
synthetic chemical toxins: insecticidal action of naturally occurring vegetable
products like pyrethrum, rotenone, but above all products of the neem tree –
Fungicidal action of olive oil production residues („Schädlingsbekämpfung unter Vermeidung synthetischerchemischer Gifte: Insektizide Wirkung natürlich vorkommender pflanzlicher Produkte wie Pyrethrum, Rotenon, vor allem aber Produkte des Niem-Baumes. Fungizide Wirkung von Olivenölproduktions-Rückständen“),
Ms Mar 51, 25 p (unpublished)
For centuries the Indians have venerated the neem
tree as a "health donor" that protects humans against stored food and
plant pests as well as pathogens. Valuable preparations can be produced from
both the seeds (→ oil) as well as from the leaves (- - > aqueous and /
or alcoholic extracts) which in
It became known in
(14) R Riemschneider
"Re-reading 66 years of chemistry" (“Nachlese – 66 Jahre Chemie”) with approx 1,500 references (own publications, lectures, lab reports, patents) and descriptions of PROJECTS I - XXVI plus vita (in preparation)
(15) R Riemschneider
Bull R12, Inst of Physiolog Chem, Univ of Berlin, Dec
47, 12 p, extracts published in 9th spec issue, 1st suppl
vol of Pharmazie 1949, p 755, 769, 789, from 16 - 18, 21, 30, 34 - 41; also
Chim et Ind (Paris) 1950, 695 - 698. Cf W Cohnen in Seifen, Fette Öle, Wachse 1950, 277. Revs: Chem Abstracts 48
2973; Angew Chem A 60, 70 (1948); Dtsch Ges Wes 3 718 (1948); Natur und
Technik H 15, 173 (1948); Chemical Age 59 (July, Oct 48); Pharmaz 3 506
(1948); Seifen, Öle, Fette, Wachse 74 80 (1948); Chem Zbl 344, 531 (1948
II)
(16) R.
Riemschneider
Chemistry of Polyhalocyclopentadienes, Bull
XXXV: The Chemistry of the insec- tizides
of the DIEN-group, (Polyhalocyclopentadien-Chemie,
Mitt. XXXV: Die Che mie der Insectizide der DIEN-Gruppe).
World Review of Pest Control, Vol 2 29-61
(1963).
For correspondence please use our e-mail
account: rriemschneider@yahoo.de
[1] The author had
already warned against banning the use of DDT in several lectures in 1948-50
[eg (4b, d)]; one could not imagine the consequences of a complete ban after a
certain amount of years, he said. In fact DDT was banned globally in the
sixties and already very short time afterwards, namely in 1969 there were again
more than 2 million cases of malaria in
[2] Quotations of publications from the years 1945-47
to be found in the list of references in (15).
* eg: lines 1, 3, 5,
7 etc in one letter and lines 2, 4, 6, 8 etc in a second. After some letters
were seized, we worked with three letters for one text, posted in different
towns with differing senders
[ BWW Society Home Page ]
© 2005 The BWW Society/The Institute for the Advancement of Positive Global Solutions