Ortho-Diacetylbenzene (o-Di) and Some
Analogues in Amino Acid Analytics and as Marker in Criminalistics
o-Di Competitor of Ninhydrin?*)
by Prof Dr Dr Randolph Riemschneider
Institute of Biochemistry, Free University of Berlin
(FUB), Germany
and
Central Institute of Chemistry,
Universidade Federal de Santa Maria (UFSM), Santa Maria, Rio Grande do Sul,
Brazil
Dedicated to Prof Dr Conrad WEYGAND († 1945), who
introduced the author to the subject in 1937.
The author developped the chemistry of o-diacetylbenzene from the beginning
until Finished-product
stage ( sales catalogues, SCHUCHARDT,
MERCK) and made possible its application in analytical chemistry as a ninhydrin competitor and its use as a
marker in criminalistics. The study of o-Di-analogues resulted into a kind of Polyacyl Chemistry: Table 1,2 and Plate 1.
Isolation and identification of
ninhydrin-reductone brought light into the ninhydrin amino acid reaction: Plate
4 and 3.
The editors
The author first prepared o-diacetylbenzene (o-Di) in 1937, using a
special oxidation method, namely the permanganate oxidation of o-ethyl
acetophenone in buffered solution (1, 11). o-Di is a white powder, from m p 39
- 40oC, which can be ground to an almost invisibly fine dust and
stains the fingers dark-blue/violet on contact. An indelible dye begins to form
some 10 minutes after contact. We took considerable interest in this
interesting substance and some of its analogues with neighbouring acyl groups
on ring systems, primarily aromatic ones; cf Tab 1 and 2, Pl 1 in appendix.
_______________
The readily
visible, intensive staining by o-Di with amino acids and proteines suggested:
1) to test the compound for
analytical applicability and whether it could compete with the accepted amino acid reagent
ninhydrin
2) if so, to find a simple and
practicable pathway for synthesizing o-Di
3) parallel to this, to seek among
o-Di analogues compounds which, like o-Di and ninhydrin,
are characterized by neighbouring carbonyl functions on aromatic ring systems
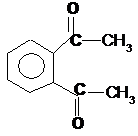
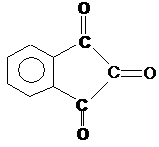
o-Diacetylbenzol Ninhydrin[1])
1) The analytical chemistry of o-Di
Both o-Di and
ninhydrin are well suited for determining the concentration on amino acid
solutions by colorimeter: Tables 3
and 4 in appendix. Ninhydrin is more sensitive for chromatographic determinations, the dyestuffs form o-Di and amino
acids in solution are characterized by greater stability (2,3,4,)[2])
Experience in amino acid analyser present in (5).
The continuing investigations into the use of o-diacetylbenzene[3])
as a fluorescent reagent for
determining amines, amino acids and proteins showed that o-Di is well-suited in
the case of histamine, glutamine, ornithine, lysine, and taurine: Lecture in
July 1958 (6a).
Dyes from o-Di and histamine (glutamine, lysine) were isolated and
analyzed in collaboration with Dr H Höllriegel, and Dipl-Chem H-J Hein in 1980,
working at temperatures below -25°C in organic solutions, in varying pH
conditions and using modern physical methods (6b).
Our fluorescence-microscopic
investigations with o-diacetyl-benzene into fixed paraffin sections of guinea
pig organs and frozen sections of fresh tissue showed that the tissue protein
NH2-groups are linked to the reactions with o-Di: there is no fluorescence reaction if the NH2-groups
are blocked; blocking the COOH-groups increases the fluorescence reaction (7,8).
Despite great efforts[4],
we did not succeed in clarifying the mechanism of color reactions of o-Di with
amino acids, but were able to make progress with respect to the mechanism of
the ninhydrin reaction: cf. plate 4 in appendix.
The familiar ninhydrin was included
in our investigations of the color reactions of cyclic di- and tricarbonyl
compounds with amino acids, peptides, proteins, and amines. In this way, we
came to renewed investigation of color reaction of ninhydrin with amino acids.
Modification of ninhydrin
reaction mechanism postulated[5]) (10 a,b,c):
Starting from the postulated mechanism of ninhydrin reaction with amino
acids, we initially endeavored to recover and identify an expected tautomeric reaction intermediate: Ninhydrin Reductone (II
a ⇋ II b) received namely by alkaline saponification of
2-acetoxy-indandione-(1,3) (III) in ultrapure nitrogen: Plate 2 in appendix (10a).
By reason of our investigations into the reductone II b, we have
formulated the ninhydrin reaction since 1960 as in Plate 4. This plate is taken
from the author’s “Material for biochemical introductory lectures” 1969, 1st
ed, p 43 (10c,e).
The fact that with ninhydrin the individual amino acids yield variously
colored products is probably linked, inter alia, to the lability of the
reductone formed as an intermediate substance. To what extent dyestuffs of the
type X formulated in (10d,e), as obtained by us when we excluded water,
requires further investigation.
2) Preparation of o-Di (1,
11,14, 15, 30)
We have
experimentally tested many possibilities for preparing o-Di over the years and
reported on them: Plates 4 and 5 in appendix.
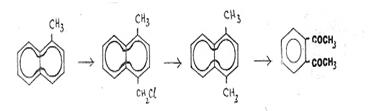
The
oxidative degradation of 1,4-dimethyl-naphthalene (C12 > C10)
proved
to be the best preparative pathway for recovering o-Di once we had improved
synthesis conditions for producing the starting materials (14).
1-methyl-naphthalene
(C10H7CH3)
↓
1-chloromethyl-4-methyl-naphthalene
(C12H11Cl)
↓
1,4-dimethyl-naphthalene
(C12H12)
↓
o-Diacetylbenzene
(C10H10O2)
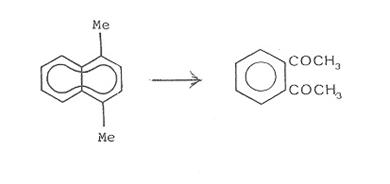
At the
request of Schuchardt Co in
3) o-Di Analogues: „Polyacyl Chemistry“ – a
further 50 years of research in the field of acyl compounds
The
bibliography in (13) shows that in the course of the following 50 years since
1937 numerous publications appeared – as we casually say today – about
“polyacyl compounds”, poly refering in this case to some triacetyl-, one
tetraacetyl- and one hexaacetyl compounds. It proved impossible to realize the
long striven for pentaacetylbenzene.
Many of
the diacyl- thru tetraacyl compounds prepared are set out below in two tables
and some plates, namely: in Tab 1 (App) those with at least one ortho position
of acyl groups on the aromatic ring system; in Tab 2 most of the acyl compounds
synthesized till 1960; in Pl 1 those obtained by diene synthesis from cis-
and/or trans-diacetylethylene and unsaturated compounds (29, 28).
Only
after some 30 years did it prove possible to obtain a homologue of
o-diacetylbenzene crystallized pure,
namely to prepare 1-acetyl-2-propionyl-benzene (II) by oxidative degradation of
1-methyl-4-ethyl-naphthalene. See Tab 1: II,
mp 49-50°C (16,17,18).
In
earlier years we had only obtained II
as oil from bp at 13 mm 148-150°C (16) namely on the following synthesis
pathway: phthalic anhydride, phthalyl acetate, 2-acetyl-benzoic acid, methyl
phthalide, 2-ethyl-benzoic acid + propionic acid (over ThO2 at
400°C), o-ethylpropiophenone, oxidized with AgMnO4 to II-crude product (staining hands): Ref
(1).
4) The applications and incidents with o-Di over the years were varied and at
times strange:
Criminalistics,
telephone receiver affair, treatment of psoriasis, “cosmetic” applications of
o-Diacyl compounds (tattoos).
CRIMINALSTIC
APPLICATION (from 1947)
On
Sundays, a member of staff in the
On
Monday, the individual concerned kept his blue-violet fingers for quite a long
time and let the bottles well alone from then on.
This
was the first criminalistic application. For several years, the detectives in
TELEPHONE
RECEIVER AFFAIR
o-Di as
“avenger”: As is evident from the numerous bulletins in Acylderivate cyclischer
Verbindungen, several students and postgraduates worked in our labs at any one
time, especially 1958-62.
Among
the students with completely other subjects there was one who was conspicuous
by his malevolence and cynicism. For example, he said to Kassahn, who had been
working on o-Di for years: “I know a good synthesis, but I’m not going to tell
you it.”
This
was, of course, not the case but the barb hurt, particularly as the individual
concerned was inconsiderate towards his colleagues in other respects as well,
eg when solvents were delivered, everything for him before the others even
noticed. (note: at that time everything was hard to get - even for money – (Particular
circumstances in postwar period of occupied
He was
one unpleasant ‘customer’ among some 20 staff. Wolfgang S., known as a joker
and pornographer, thought out a punishment: the receiver of the phone in my
office was dusted with o-Di. Then the individual concerned got an outside call
and was told to hold the line. So he waited a while, got o-Di on his ear – that
was colored violet for weeks.
The
“punished” guy went to the police, who just laughed at him, particularly as he
could not name the miscreant. Public mischief – he did not suspect Wolfgang
Schneider who was working on his thesis in a completely different field that
was in any case secret)[6].
The
“punished” colleague so refrained from annoying others from then on.
“TREATING”
PSORIASIS
A
colleague named KOKA from
We
pursued this approach in several directions:
Initially
with a number of polyacyl compounds that colored less strongly but still
reacted with proteins. Parallel to this, we conducted systematic experiments
with esters of fumaric acid:
HC-COOR
║
ROOC-CH R
= allyl, alkyl, aryl, aralkyl, alkynyl, alkenyl
This
pathway was more promising and soon led to a commercial preparation, in 1958.
Between 1958 and 1960 we succeeded in finding highly skin compatible fumaric
acid esters that permitted successful treatment of psoriasis. Experience with
over 500 patients was gained in collaboration with Japanese and Brazilian dermatologists:
1958-63.
TATTOO
EXPERIMENTS
o-Diacetylbenzene
(I) and 4,5-diacetyl-cyclohexene-(1) (II) were subjected to intensive
pharmacological and toxicological testing before we had practical tattoo experiments
conducted on mammals and then on humans too (19). The documentation on the
experiments, conducted in Brazil, is deposited with Consulting-Development-Engineering
of São Paulo. No application for doing tattoos with I and II on humans has been
submitted in Germany. In Brazil, tattoos with I and II were only done under
medical supervision.
It is of great interest that temporary tattoos are possible (19). The
dermatological and toxicological tests done to date, as well as the Ames test,
make practical use seem possible.
APPENDIX: Tables 1-4, Plates 1-6
Table
1: o-acylated benzene and
toluene derivatives and
o-diacetylnaphthalene
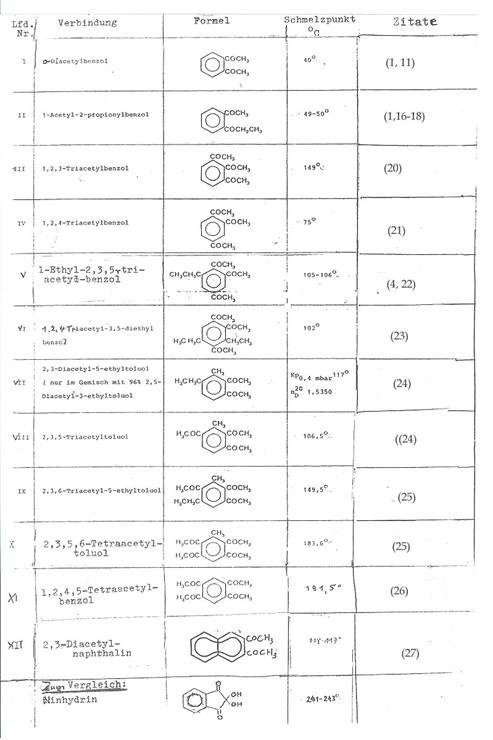
Table 2: Cyclic compounds with 2 to 3
carbonylfunctions (1937-62)[7]
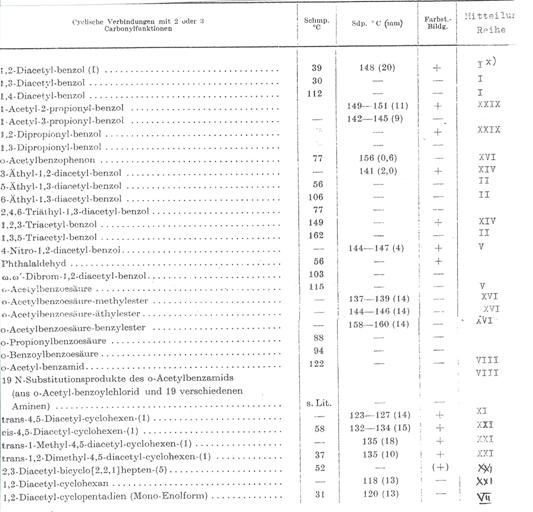
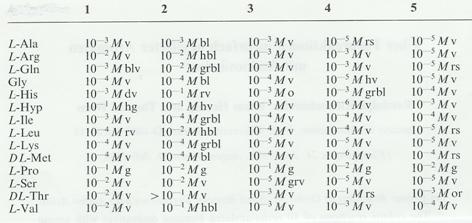
Table 3: Color reactions of o-acyl compounds with
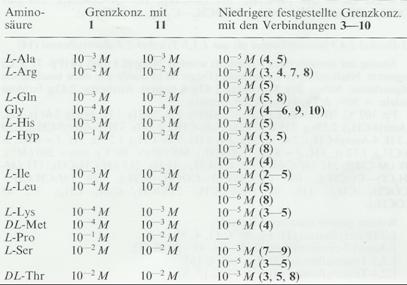
amino acids: Limiting concentration and coloration
Key: 1 o-diacetylbenzene, mp. 40°C
2 1-acetyl-2-propionyl-benzene,
mp. 49-50°C
3 1,2,3-triacetyl-benzene,
mp. 149°C
4 1,2,4-triacetyl-benzene,
mp. 75°C
5 1-ethyl-2,3,5-triacetyl-benzene,
mp. 105°-106°C
6 1,3-dietyhl-2,4,5-triacetyl-benzene,
mp. 102°C
7 1-methyl-3-ethyl-5,6-diacetyl-benzene
(nur im Gemisch mit 96% 1-methyl-3-ethyl, 2,5-
diacetyl-benzene, bp 117°C (0,4Torr), n: 1,5350 (20°C)
8 1-methyl-2,3,5-triacetyl-benzene,
mp 106,5°C
9 1-methyl-3-etyhl-2,5,6-triacetyl-benzene,
mp 149,5°C
10 1-methyl-2,3,5,6-tetraacetyl-benzene,
mp 183,6°C
for
comparison:
11 ninhydrin, mp
241° - 243°C
legend of colours (pH:
8,00): bl blue; gr grey; or orange; br
brown; gn green; r red; d dark, h (hell) light, rs (rosa) pink, g (gelb) yellow, o
(olive), v violet.
Legend to table 3:
Color reactions on spot plates: 14 amino acids were dissolved in
concentrations of 10 -1 to 10 -6 M in Kolthoff buffer pH
8.0 (10.1 g borax/l (a); 13.62 g KH2PO4 / litre (b));
buffer solution: 53.5 ml a) and 46.5 ml b). The test reagents were 0.01 molar
in 96% ethanol. - 100 μl each of amino acid solution and reagent solution
were pipetted into test tubes, where some of the more concentrated amino acid
solutions colored after a few minutes. The blends that were still uncolored
after being left to stand for 30 mins were heated to boiling over a low flame
in order to volatilize the ethanol, resulting in coloration in further
mixtures. The limiting concentration of the amino acids was determined by
transferring still uncolored or very weakly colored solutions on to spot plates
and heating in the drying oven to 80°C till dry (approx 30 mins). Limiting
concentrations and colorations are set out in the table.
In part other colorations were
observed at pH values other than 8.0
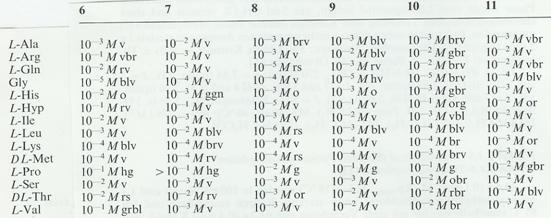
Table 4: Increasing the detection sensitivity of amino
acids as compared with the standard
reagents o-diacetylbenzene (I) and ninhydrin (II)
Key to Tab 4: List of substances more sensitive to a specific amino acid
than the standard reagents I and II (key as in Tab 3)
Plate 1: The o-diacetyl compounds I- XIV
obtained from DIEN synthesis (28, 29)
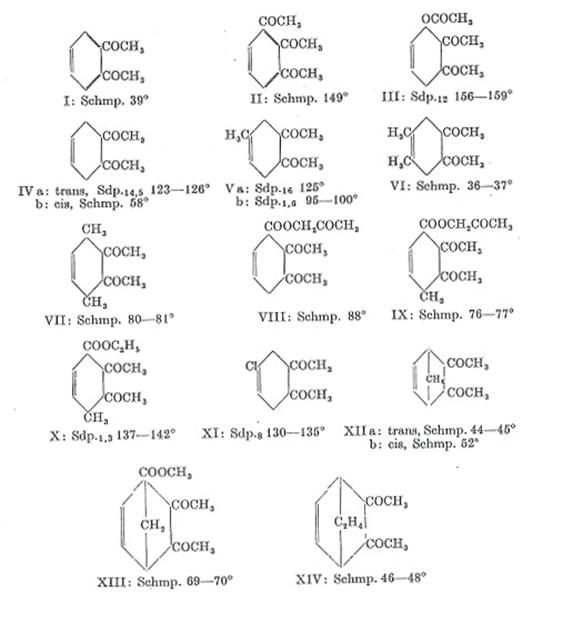
Plate 2: “Ninhydrin-Reducton” [IIa tautomer
IIb] (10a) 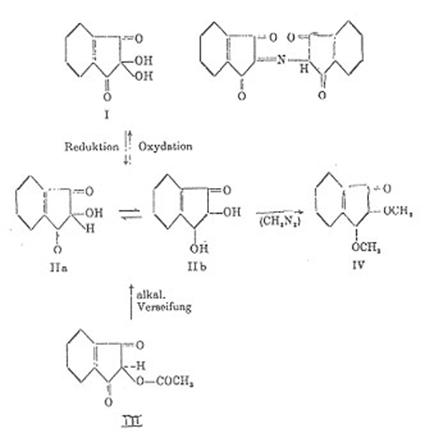
Legend to plate 2:
The structure of the reductone II b was
proved in 1960 as follows (previously unpublished) Lecture on 4 Sept 1960 in
Tokio (10a) and on October 1960 in Höchst (10b)
Aqueous solutions of II b are colored reddish-orange. The action of O2
results in decolorization while forming ninhydrin; the reaction can be followed
by spectroscope.
Reductone characteristics:
Tillmann reagent (acetic acid dichloro-indophenol solution) is decolored
by II b, AgNO3 solution immediately reduced.
Dimethyl ether mp 95°C (IV) results from
IIb with diazomethane; cf also Bull I in “On knowledge of ninhydrin reactions”
(10a).
Quantative determination of II b is possible by indirect iodometric
titration.
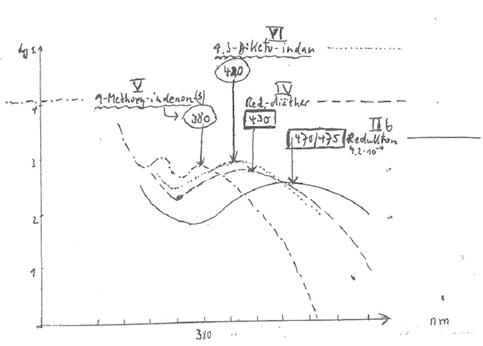
Comparison of the UV spectra peaks of II b, IV, V and VI in
ethanol by strictest exclusion of oxygen showed: Fig 1: (10a,b)
II b 1,2-dihydroxy-indenone-(3) 475 nm 4
x 19-4 mol/l [8])
IV 1,2-dimethoxy-indenone-(3) 430 nm 2
x 10-4
V 1-methoxy-indenone-(3) 380
nm 5 x 10-4
VI 1,3-diketo-indane 420 nm 7
x 10-5
Both II b and VI are largely enolized and dissociated
in alcohol/water, resulting in a strong bathochromic shift up to 40 nm in the
mesomerism of the compounds vis-à-vis IV and V. Monoenolates from II b and VI:
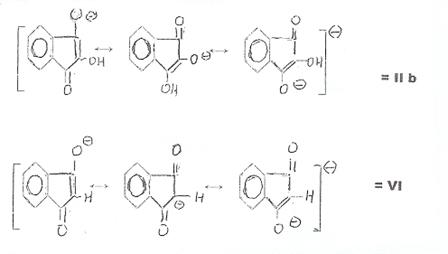
fig.1:
UV-Spektren von IIb, IV, V und VI
IR
spectroscopic investigations (10e)
KBr
pressed disks with 2 mg/g KBr
The author discussed the IR spectra of II b, IV, V and VI in detail in
the lectures of Sept 60 and Oct 1960 (10b,
c, e):
The peaks of CO valence vibration for the enol ethers IV and V are at
1701 - 1702 cm-1. For VI, two band maxima occur, namely at 1704 cm-1
and 1740 cm-1; for II b at 1748 and 1720 cm-1. Wave
number of C=C vibrations of V maximum at 1564 cm-1, corresponding
band of IV at 1600 cm-1. IV and V show split bands at 1630/1941 and
1605/1617 cm-1, stronger for diether. Deformation vibrations of H
atoms of methyl groups of IV and V: in the range of 1460/1468 cm-1.
Plate 3:

Plate 4: Reformulation of ninhydrin reaction (10a-d)
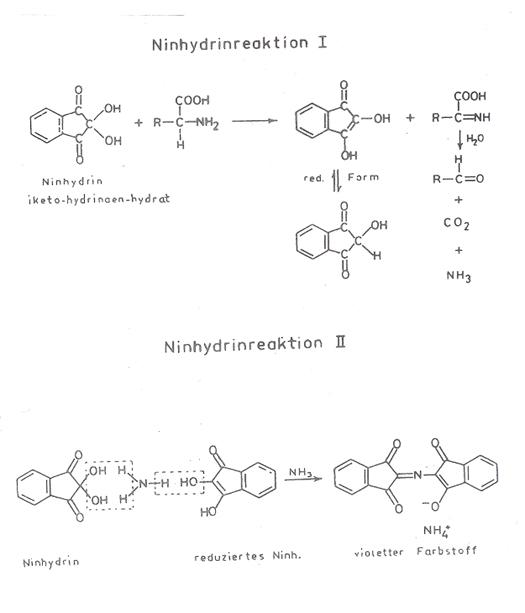
Plate 5: Synthesis
pathways to o-Diacetylbenzene adopted in own work (13)
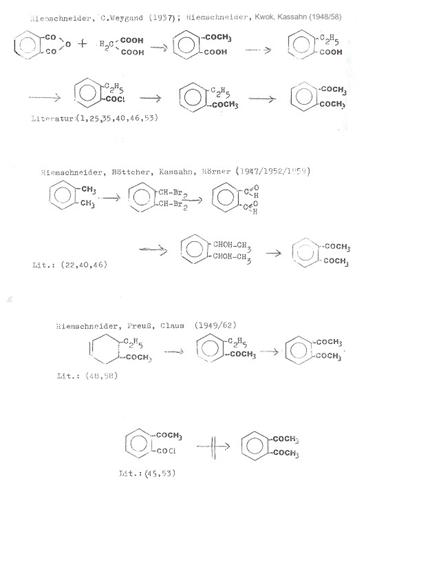
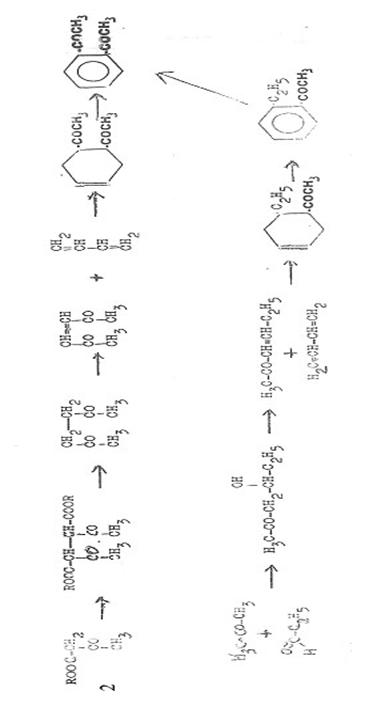
Plate 6: Synthesis pathways to
o-Diacetylbenzene adopted in own work
(13)
References:
(1) R Riemschneider
Oxidation of ethylbenzene, o-,m-,p-diethylbenzene
and o-ethylacetophenone with potassium permanganate in buffer solution: o-diacetylbenzene[9]) -
Permanganate oxidation in organic solvents[10])
(Oxydation von Ethylbenzol,
von o-,m-,p-Diethyl-benzol und von o-Ethylacetophenon mit Kaliumpermanganat in
gepufferter Lösung: o-Diacetylbenzol – Permanganatoxidation in organ. Lösungsmitteln).
Voluntary Year’s Paper in chemistry for
graduation at Matthias Claudius High School in Hamburg-Wandsbek, done from June
1937 till December 1938, submitted in Jan 1939: ms 96 pages.
Consultants for Year’s Paper in chemistry were: Dr W Hirsch (chemistry)
and R Bach (mathematics and physics). Professor Dr H H Schlubach, Organic
Chemistry at University of Hamburg, also gave an expert opinion - Summary
of some results published 1947 as bulletin I in series “Acylderivate cyclischer
Verbindungen”, deposited on 12 Sept. 1940[11])
with editors of GAZZ. CHIM. ITAL., Rome: (11).
(2) R Riemschneider, C Weygand †
Bull III: On the suitability of 1,2-diacyl-benzenes for proving and
determining of amino acids (Über
die Eignung von 1,2-Diacyl-benzolen zum Nachweis und zur Bestimmung von
Aminosäuren)
Mh Chem 86, 201 - 209 (1955)
Prof Weygand was drafted into the Volkssturm (Hitler’s last-ditch levy)
and fell in February 1945, shortly before the end of the second world war. He
is cited as coauthor in gratitude for introducing me to the subject, and in
reverence.
(3) R Riemschneider, J Wierer
Bull XII: o-Diacetylbenzene as amino acid reagent – a comparison with
ninhydrin (o-Diacetylbenzol
als Aminosäurereagens – ein Vergleich mit Ninhydrin)
Z analyt Chemie 193, 186-189 (1962)
(4) R Riemschneider, K Hennig, T Wons
Bull XXVII: Color reactions of polyacylated aromatics with amino acids (Farbreaktionen mehrfach
acylierter Aromaten mit Aminosäuren)
Mh Chem 118, 831-835 (1987); see table 3 and 4.
(5) R
Riemschneider
Bull XXXVI: “Application of o-diacetylbenzene and
1-ethyl-2,3,5-triacetyl-benzene as color reagent in amino acid analyser” (Einsatz von o-Diacetylbenzol
und 1-Ethyl-2,3,5-triacetyl-benzol als Farbreagens im Aminosäureanalysator)
Ms 1985, 6 p
Part of lecture given in colloquium of Institute of Chemistry, SAITAMA
University, Urawa, Japan on 23 August 1985, (chair: Prof Dr J T Shimozawa)
(6a) R Riemschneider (lecturer), M Somplatzki, H G Kassahn, T H Kong
“o-Diacetylbenzene as
reagent for fluorescence, above all for histamine, ornithine, lysine, taurine.
Selective determination of L-lysine” (o-Diacetylbenzol
als Reagenz für die Fluoreszenz, vor allem für Histamin, Ornithin, Lysin,
Taurin. Selektive Bestimmung von L-Lysin)
Ms 1958, 14 p
Lecture given in colloquium of Department of Biochemistry at Free University of Berlin
Manuscript replicated (100 copies distributed to students)
Starting point for experimental results presented was early (1948-49) investigations (7,8). Instructions for determining histamine, glutamine, ornithine, lysine and taurine. Ibidem also with regard to o-phthalic aldehyde reaction with amino compounds.
(6b) R Riemschneider, H Höllriegel, H-J Hein
Dyestuff formed from o-diacetilbenzene and histamine, glutamine, lysine.
Isolation and analysis of dyestuffs formed at -25°C, in large scale experiments, from o-diacetilbenzene and histamine (glutamine, lysine) by extraction, lyophilisation, followed by a series of chromatographic steps, by semipreparative high performance liquid chromatograohy, NMR and mass-spectroscopy
Ms 1980, 12 p (microfilm, in
possession of Dipl.- Chem. H. J. Hein)
(7) R Riemschneider, A Küchenmeister, M
Somplatzki, C Winter
Fluorescent microscopy experiments with o-diacetylbenzene on mounted
paraffin sections of guinea pig at pH 9.0 (Fluoreszenzmikroskopische
Versuche mit o-Diacetylbenzol an
fixierten Paraffinschnitten von Meerschweinchenorganen bei pH 9,0.)
Lab reports 1948, 10 p plus appendix to Somplatzki’s 1952 diss.
Flourescence evident after approx 1 min. Lasts 4
- 6 mins with 0.15% o-di diluted 1 : 800 (ZEISS luminescence apparatus).
The short-lived light-blue fluorescence is bound to NH2-groups
in tissue, as various blocking tests show: fluorescent compound(s) stable for
short period only as a function of pH and then change(s) into familiar colored
product(s).
No fluorescent reaction when NH2-groups blocked by acylation
but increased reaction if acid groups blocked by esterification.
(8) R Riemschneider, M Becker, M Somplatzki
Fluorescence microscopy experiments with o-diacetylbenzene on frozen
sections of fresh tissue from South African clawed frog Xenopus laevis (Fluoreszenzmikroskopische
Versuche mit o-Diacetylbenzol an Gefrierschnitten von frischen Geweben des
südafrikanischen Krallenfrosches Xenopus laevis)
Lab reports 1949, 6 p
Strong light-blue fluorescence after 2 mins, stable approx 20 mins, then
changes into familiar blue coloration (fluorescence fades).
The experiments to (28, 29) were conducted in the Institutes of
Physiology and Physiological Chemistry of University of Berlin from 1947 -
beginning 1948. Thanks to Prof Dr E Fischer, Deputy Director of Institute of
Physiology, for invaluable advice on how to conduct these experiments.
On the advice of Dr Siebenmarck of the University of Leipzig, the two
lab reports (7,8) were sent via Prof Dr E Fischer to Prof Dr H Voss, Director
of the Institute of Anatomy, University of Jena, as he had been interested in
the color reaction of o-diacetylbenzene during the second world war already
(discussions with Weygand, Siebenmarck and Voss). Unfortunately, Wartenberg and
Voss made no reference to the experimental data sent to them in their later
publications. It must be assumed that Cold War political realities played a
part, as E Fischer and the author were working in the West at the Free
University of Berlin from 1950.
(9) R Riemschneider, H Koch, Y Morino
o-Phthalaldehyde for detection and determination of amines and amino
acids: formation of strongly fluorescent derivatives (o-Phthaldialdehyd zum Nachweis und zur Bestimmung von Aminen und
Aminosäuren: Bildung stark fluoreszierender Derivate)
Ms 1947, 16 pages (unpublished)
Most favorable conditions established for producing and isolating dyestuff
from o-phthalaldehyde and histamine at -10 – -20°C are described in more detail
at (13).
(10a) R Riemschneider
Bull I: “On ninhydrin and the tautomerism of bis-1,2-hydroxy-indanone-(3) with 2-hydroxy-indandion-(1,3)” (Über Ninhydrin und die Tautomerie des Bis-1,2-hydroxy-indanon-(3) mit dem 2-Hydroxy-indandion-(1,3))
Mh Chem 93, 841-842 (1962)
Presented to Japanese Chemical Society in Tokyo in Sept 1960
(10b) R Riemschneider
Bull II: “On knowledge of ninhydrin reaction. Summarized presentation of ninhydrin reaction with regard to own experiences and investigations” (Zur Kenntnis der Ninhydrin-Reaktion. Zusammenfassende Darstellung über die Ninhydrin-Reaktion unter Berücksichtigung eigener Erfahrungen und Untersuchungen)
Lecture in chemical colloquium of Farbwerke Hoechst in Frankfurt-a-M in October 1960, at instigation and invitation of Head of Department, Dr F Scherer
(10c) R Riemschneider, R Koka, H Kieseler
Bull III:
Reaction of triketohydrindene with “labeled” α-amino
acids under exclusion of water (Umsetzung von
Triketohydrinden mit „markierten“ α-Aminosäuren unter Ausschluß von
Wasser)
Mh Chem 94, 1131-1132 (1963)
(10d) R Riemschneider
Material for
introductory lectures on biochemistry
at Free University of Berlin 1969, p 43: Reformulation of ninhydrin
reaction („Material
für biochemische Einführungsvorlesungen“),
Freie Universität Berlin 1969, 1. Auflage, Seite 43: Neuformulierung der Ninhydrin-Reaktion) (based on results of Bull I and IV); cf. PROJ: I and XXVI in (13).
(10e) R Riemschneider, H Kieseler
Bull IV: On knowledge of ninhydrin reaction. Further experimental data on results of Bull I – III (Zur Kenntnis der Ninhydrin-Reaktion. Weitere experimentelle Daten zu Ergebnissen der Mitt. I bis III)
Ms 1963, 60 p
(10f) R Riemschneider, J Wierer
Bull V: Comparison of ninhydrin and o-diacetylbenzene as reagent for amino acids and amines (Vergleich von Ninhydrin und o-Diacetylbenzol als Reagens für Aminosäuren und Amine)
Z analyt Chemie 193, 186-189 (1962); see also table 3 and 4.
(11) R
Riemschneider
Mitt I: Derivati
acetilici di combinazioni isocicliche: o-, m-, e p-diacetil benzolo
Gazz Chim Italiana 77, 607 - 611 (1947), date of receipt 12 September 1940, deposited in agreement with Prof
C Weygand, University of Leipzig.
Reason for the late release
for publication; cf. also footnote 11 to
(1) and PROJ I 2 in (13)
At
his express wish, Prof Weygand is not cited as coauthor as the results pertain
to investigations conducted externally [Year Paper of Chemistry, Matthias
Claudius High School, Hamburg (1)]
(12a) R Riemschneider (lecturer), H-J Hein, H Kahl
“Permanganate oxidations in organic solvents (acetic
anhydride, pyridine (AgMno4), glacial acetic acid), use of permanganates of
potassium, sodium, lithium, barium, strontium, calcium, aluminum and silver for
permanganate oxidation.” (Permanganatoxydationen in
organischen Lösungsmitteln)
Summary of lecture given end of July 1943 in chemical colloquium of
works laboratory of RUHRÖL GmbH, Bottrop; ms 44 pages. Given as habilitation lecture
at Faculty of Math and Nat Sc of Kaiser Wilhelm University of Berlin on 21
January 1948, incorporating test results from 1938 to 1947 (1): certified
copies of habilitation procedures of 09 January 1950; cf also (12b).
Only
the permanganates of potassium and silver are suitable as oxidizing agents.
(12b) R Riemschneider
Habilitation colloquium at Fac of Math & Nat Sc of Humboldt
University of Berlin: “Permanganate oxidations in organic solvents” (Permanganatoxydationen
in organischen Lösungsmitteln); on 21 January 1948; cf also (1,12a).
Confirmation of habilitation in writing by Prof Dr K Lohmann on 3 March
1948.
(13) R.
Riemschneider
Re-reading - 66
years of chemistry (Nachlese – 66 Jahre Chemie) with approx 1,500 citations (own
publications, lectures, lab reports, patents) and descriptions of PROJECTS I -
XXVI plus vita
(in
preparation). Here relevant PROJ. I.
(14) R Riemschneider, K Nolde, K Hennig
Bull XXII: Notice on preparation of diacetylbenzene (Notiz
zur Darstellung von o-Diacetylbenzol)
Mh Chem 104, 987-989 (1973). Schuchard Co (Munich) produced the o-di
offered in its sales catalog according to this prescription since 1960: plate 3,
later adopted by Merck (Darmstadt)
(15) R Riemschneider
Bull IV: o-Diacetylbenzene derivatives (o-Diacetylbenzol-Derivate)
Patent registration R 21633 IV b/12b of 05 August 1957,
(16) R Riemschneider, D Y Kwok
1-Acetyl-2-propionyl-benzene from [phthalic anhydride, 2-acetyl-benzoic
acid, methylphthalide, 2-ethyl-benzoic acid + propionic acid (via ThO2
at 400°C)], o-ethyl-propionophenone, oxidized to acetyl-2-propionyl-benzene
bp 148 - 150°C (13 mm).
Mitt Physiolog-chem Institut Berlin, 1948, 11 pages.
(17) R
Riemschneider, T Wons
Bull XXIX b: 1-Acetyl-2-proprionyl-benzene, m.p. 49°-50°C
Presented to J Am Chem Soc (received in Columbus, Ohio on 28 May 1985):
Table1.
(18) R
Riemschneider (lecturer), T Wons
Bull XXIX a: “Homologues of o-diacetylbenzene: 1,2-diproprionyl-benzene
and 1-acetyl-2-proprionyl-benzene” (Homologe des o-Diacetylbenzols:
1,2-Dipropionyl-benzol und 1-Acetyl-2-propionyl-benzol)
Ms 1985, 6 p; cf lecture (31)
(19a) F R Pesserl, R
Riemschneider, H Fereira
Bull XLVI: Continuation of investigations from Bull XLIII:
1985-90/1999-2003 long-term experiments to test for carcinogenic effect
of o-diacetyl-benzene and 4,5-diacetyl-cyclohexene (skin of rats, pigs), simultaneous
continuation of practical tattooing tests (temporary tattoos), Ames tests (Langzeittestversuche
zur Prüfung auf kanzerogene Wirkung von o-Di und 4,5-Diacetylcyclohexen:
1985–1990, 1999-2003 (Rattenhaut, Schweinehaut), gleichzeitig auch Fortsetzung
der praktischen Tätowierungsversuche (zeitlich begrenzte Tätowierungen),
AMES-Teste)
Ms 1991 (unpublished)
Tests conducted in labs of Consulting-Development-Engineering in Sao
Paulo, Brazil
Results: o-diacetylbenzene shows no mutagenic effect; it is suitable for
tattooing.
(19b) R Riemschneider, M
Salvioni
Bull XXXIV: Application of diacetylbenzene (I) and some analogues, for
instance 4,5-diacetyl-cyclohexene isomers (II) as dye stuffs in cosmetics: long-term colorings
of skin (tattooing) toxicol. expriments of I and II. Experiences from
1985. [Anwendung
von o-Diacetylbenzol (I) und einiger Analogen, z.B.
4,5-Diacetyl-cyclohexen-Isomere (II) als Farbstoffe in der Kosmetik:
Langzeitfärbungen der Haut (Tätowierung) – Toxikolog. Untersuchungen mit I und
II . Erfahrungen ab 1985]
Ms 12 p (secreted).
(20) R Riemschneider, B Dietrich
Bull XVII: 1-Ethyl-2,3-diacetyl-benzene and 1,2,3-triacetyl-benzene (1-Ethyl-2,3-diacetyl-benzol
und 1,2,3-Triacetyl-benzol)
Liebigs Ann Chemie 646, 18-23 (1961).
(21) R
Riemschneider, T Wons
Bull XXIV: 1,2,4-Triacetyl-benzene from
6-acetyl-1,4-dimethyl-naphthalene (1,2,4-Triacetyl-benzol
aus 6-Acetyl-1,4-dimethyl-naphthalin)
Mh Chem 114, 1267-1268 (1983).
(22) R
Riemschneider, K Hennig, T Wons
Bull XXVII: 1-Ethyl-2,3,5-triacetyl-benzene, an amino acid reagent (1-Ethyl-2,3,5-triacetyl-benzol,
ein Aminosäurereagens)
Z analyt Chemie 325, 561-562 (1986)
(23) R
Riemschneider, K Hennig, T Wons
Bull XXVII: Color reactions of polyacylated aromatics with amino acids (Farbreaktionen
mehrfach acylierter Aromaten mit Aminosäuren)
Mh Chem 118, 831-835 (1987)
(24) R
Riemschneider, K Hennig
Bull XXVI: 2,3,5-Triacetyl-toluene (2,3,5-Triacetyl-toluol)
MhChem116, 873-876 (1985)
(25) R
Riemschneider, K Hennig
Bull XXV: 2,3,6-Triacetyl-5-ethyl-toluene and
2,3,5,6-tetraacetyl-tuluene (2,3,6-Triacetyl-5-ethyl-toluol
und 2,3,5,6-Tetraacetyl-toluol)
Z Naturforschg 39 b, 835-838 (1984)
(26) R Riemschneider
Bull XLI: 1,2,4,5-Tetraacetyl-benzene (I) thru
oxidation of 1,4-dimethyl-6-ethyl-7-acetyl-naphthalene and/or thru oxidation of
1-ethyl-2,4,5-triacetyl-benzene (II) [ I ← II
] (1,2,4,5-Tetraecetyl-benzol
(I) durch Oxydation von 1,4-Dimethyl-6-ethyl-7-acetyl-naphthalin bzw. durch
Oxydation von 1-Ethyl-2,4,5-triacetyl-benzol
(II) [ I ← II ].
Ms December 1985, 9 p

Proof of structure for I and II thru MS, IR, NMR
Experiments conducted in Central Institute of Chemistry at the Federal
University of Santa Maria (UFSM), Santa Maria, Rio Grande do Sul, Brazil
(27) R Riemschneider (lecturer), H-J Hein
Bull XVIII: “o-Diacetylnaphthalene through a) degradation of 1,4-dimethyl-anthracene
b) synthesis, starting from naphthalene-dialdehyde-(2,3)” [o-Diacetylnaphthalin, a)
durch Abbau von 1,4-Dimethyl-anthracen, b) durch Synthese, ausgehend von
Naphthalin-dialdehyd-(2,3)]
Lecture of 12 November 1962: “o-diacetylnaphthalene (I)
[2,3-diacetyl-naphthalene] mp 118°C” in chemical colloquium of
University of Santa Maria (USM), S Maria, RS, Brazil
Ms January 1961, 12 p, 100 hectographed copies distributed (in English)
as manuscripts.
Extract from lecture manuscript: The constitution of
2,3-diacetyl-naphtalene (I) m.p. 118-119° C, was proven by
IR-spectroscopy (fig.) and by colour reactions of I with amino acids (tabl).
Colours observed: dark violet with Gly; violet: Ala, Phe; red/violet: Ser, Lys,
β-Ala, Leu, Thr, Pro; blue: His; yellow-green: Try. The formation of
oximes and hydrazones was disturbed; the high activity of CO-groups results in
polymerization and aldol condensation: the IR-spectrum shows OH-bands.
The starting material, the diol, oxydized to I (via
b), is melting at 131°C, its dibenzoat at 153°C.
(28) R
Riemschneider, K Preuß
Bull XI: Color reactions of amino acids with 4,5-diacetyl-cyclohexene-(1), [Farbreaktionen
von Aminosäuren mit 4,5-Diacetyl-cyclohexen-(1)]
Mh Chem 90, 924-928 (1959): plate 1
(29) R Riemschneider (lecturer), P Claus
Bull XXI a: “Color reactions with cyclic di- and triacetyl compounds,
received by DIEN-synthesis” (Farbreaktionen mit cycl. Di-
und Triacetyl-Verbindungen): plate 1
Mh Chem 93, 844-850 (1962); cf also lecture of 04 September 1960 to
Japanese Chemical Society
(30) R Riemschneider, S Foerster
Bull XX: o-Diacetylbenzene from 1,4-dimethyl-naphthalene (o-Diacetylbenzol aus
1,4-Dimethyl-naphthalin)
Mh Chem 93, 616-617 (1962) For patent reasons, no refererence is made to
earlier bulletins [for instance (15)] about the synthesis of o-diacetylbenzene and
substitution products from 1,4-dimethyl-naphthalenes, ie through degradation
reactions i.e. C12 →C10 (14).
(31) R
Riemschneider (lecturer), K Hennig, T Wons, H-J Hein, G Buchlow, M Salvioni
Lecture
„Recent results in the field of the chemistry of acyl
derivatives of cyclic compounds and some side reactions” (Neuere Ergebnisse auf dem Gebiet der
Chemie der Acylderivate cyclischer Verbindungen und einiger Nebenreaktionen), given on 10 August 1984 in colloquium of Central Institute of
Chemistry at the Federal University of Santa Maria (UFSM), Santa Maria, Rio
Grande do Sol, Brazil and based on the results of bulletins XXVI - XXXI and
XXXV (13)
Lecture published in Portuguese as special edition of UFSM 1985, 21 p
(32) R
Riemschneider, K Hennig
Bull XXV: 2,3,6-triacetyl-5-ethyl-toluene and
2,3,5,6-tetraacetyl-tuluene (2,3,6-Triacetyl-5-ethyl-toluol
und 2,3,5,6-Tetraacetyl-toluol)
Z Naturforschg 39 b, 835-838 (1984)
[1] written as a triketone
[2] cf. also table 3 in appendix.
[3] also o-phthalic
dialdehyde are well suited for detecting amino acids and amines by fluorescence microscopy (9,7,8)
[4]) Involved in the investigation in o-Di reaction
with amino acids, in the course of many years (listed in chronological order)
were: M Somplatzki, H Arnold, E Fischer, P Kalb, H Oelsner, H D Otto, H Becker,
C Winter, S Painer, W Stuck, K Nolde, H-J Hein, M M Faria, F R Pesserl, H
Martins and H Höllriegel - Dipl-Chem K Nolde concerned himself especially
intensively with the coloring problem within the framework of doctoral thesis,
using spectroscopic methods. Dipl-Chem Hein and Dr W Stuck made great efforts
in the practical use of o-Di as a reagent for fluorescence and in amino
acid analyzer in 1955-58.
Unfortunately, Mr Nolde was unable to
complete these investigations as, in a secret vote, the council of the Central
Institute of Biochemistry and Biophysics at the Free University of Berlin (FUB)
refused to renew his contract as an assistant. Protests by his doctoral
supervisor (author) were to no avail during these years of "scientific destruction"
in 1969 - 75.
[5]) Results presented: “On knowledge of ninhydrin
reaction” in bulletins: I - V (10a-f).
[6] „secret“ because, the subject was one involving
the West German ministry of defence; cf
PROJECT XVIII in (13).
[7] Cf ref (13) in series of bulletins
[8] In the case of II b in ethanol/0.1 n HCl
[9] published in (11)
[10] basis for references (1, 12a,b)
[11] With
Prof Weygand’s agreement, the author had sent bulletin I entitled
“Oxidation of the 3 isomeric diethylbenzenes and o-ethylacetophenone” to the
editors of the Italian journal Gazzetta Chimica Italiana in
He decided to deposit the
manuscript for the following reason: During the authors time at school, he was
in contact with a theology student called Wonde who had already been in a
concentration camp. He had enlightened the author as to the “true intentions of
the Nazis” and advised him on no account to publish in
[ BWW Society Home Page ]
© 2006 The BWW Society/The Institute for the Advancement of Positive Global Solutions