Preparation
and Photocatalytic Activity of Nano-sized Metal
Supported TiO2-Coated
on POF
Dr. Jin-Wook Ha and Dong-sik Yu
Dept.
of Chemical and Environmental
ABSTRACT
The photodegradation of IPA according to coating conditions was
examined by TiO2-coated POF. In the photoactivity of TiO2-coated
POF, ethanol solvent was higher activity than other solvents.
Inorganic(KR-400), organic(A-9540) and inorganic∙organic hybrid(GPTMS,
TMOS) resins were used as binder. Organic binder(A-9540) showed the highest
activity for photodegradation of IPA. But organic binder was decomposed by TiO2,
inorganic binder had lower binder ability than others, but inorganic binders
were lower adhesive than organic binders. The optimum activity obtained when
the ratio of TMOS and P-25 was 0.05~1:1.
1. INTRODUCTION
Photocatalysis is attracting a great deal of attention from view
points of fundamental science and applications for practical use. The
photocatalytic degradation of pollutants using photocatalysis is attracting
considerable attention for the application to environmental problems[1-3].
Among the various photocatalytic material, TiO2 has attracted wide
interest for its potential use in environmental purification. Recently,
Photochemical degradation has been commercially applied to environmental
cleaning by utilizing photocatalytic oxidation of volatile organic
compounds(VOC) by TiO2 powers or coating[4,5]. This photocatalytic
method is based on the reactive properties of electron-hole pairs generated in
the semiconductor particles under illumination by light of energy greater than
the semiconductor band gap energy. These charge carriers can reach the particle
surface and react with species in solution with suitable redox potentials[6,7].
While various research areas on photocatalysis have been studied, the
immobilization of photocatalyst on a specific substrate, in particular,
has received considerable attention due to the versatility of application. In
this study, plastic optical fibers(POF) were considered as light-transmitting
media and substrates for the potential use in photocatalytic environmental
purification system, comparing with that of quartz optical fibers(QOF). It is
concluded that the use of POF is preferred to QOF since the advantages such as
ease of handling, lower cost, relatively reasonable light attenuation at the
wavelength of near 400nm can be obtained. And also, this system has been
identified to achieve two main goals; transfer of light and volumetric reaction
among the various immobilized reactor system[8]. In some studies, solid-gas
phase heterogeneous photocatalysis using TiO2 has attracted
considerable interest for VOC removal, in particular due to the possible use
solar radiation, and numerous applications have been proposed[9]. A large
variety of organic compounds may be oxidized by TiO2 photocatalysis
in the presence of molecular oxygen. For instance, some authors studied in a
project on air quality the photocatalyzed oxodation of oxygenated compounds,
such as acetone, 1-butanol, butanal, formaldehyde and m-xylene, detected at
working places[10]. we have been interest in the photocatalytic degradation of
aliphatic compounds bearing an alcohol and we have chosen isopropyl alcohol
(IPA) as model compounds.
In this paper, we performed deposition of TiO2-coatings
on POF, and the effectiveness of photocatalytic degradation of IPA was
investigated under several coating conditions.
2. EXPERIMENTAL
2.1. Materials
Almost Chemicals were of reagent grade and used without further
purification. Ethanol, isopropyl alcohol, tert-butyl alcohol, acetone,
1-octanol from Aldrich; TiO2 from Degussa P-25; Plastic optical
fibers(POF), Binder(Aâ-9540, KRâ-400 GPTMS; 3-Glycidoxypropyltrimethoxysilane, TMOS; Tetramethyl
orthosilicate) from commercial grade.
2.2. Preparation of TiO2-coating
slurry solution
TiO2 slurry was prepared with known amount of TiO2
in selected solvents and stirred before introducing the support. Selected
binders and diluting solvents were mixed for 1min at room
temperature. This binder mixture was added to TiO2 slurry for
40 min and were performed ball mill for 2hr. Fig. 1 shows the preparation
of TiO2-coating slurry solution.
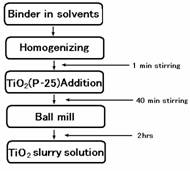
Fig.
1. Preparation of TiO2-coating slurry solution.
2.3. Plastic optical
fibers(POF) pretreatment
Pretreatment of POF could not be done either thermally or
physically because the materials of core and clad is very similar to each
other. Solvation using appropriate solvent was finally chosen to remove the
clad of POF. After removing the clad from POF with 1mm diameter, the resulted
diameter should be closed to 0.98mm(matched with data by supplier). Clear proof
of change in diameter of the POF was obtained by SEM(JSM 5900, JEOL) taken at
various time scales.
2.4. Dip coating
The POF were dipped in the slurry solution by hand in an
atmosphere. After each coated POF was allowed to dry at 80℃ for 2hr.
2.5. Experimental set-up
A schematic representation of the experimental set-up used for
performing photocatalytic degradation processes at the solid-gas interface is
given in Fig. 2. The whole configuration consists of a light source(Sankyo
Denki, 8W, BLB UV lamp, 2EA), pyrex reactor(730ml, cylinder cell type),
thermocontroller, Gas chromatography(GC: Young Rin, Column: HP-1, 30m × 0.321mm
× 0.25 ㎛, 60℃ to 325℃, Oven Temp. 30℃ , Injector Temp. 150℃, Detector Temp. 200℃), Carrier gas(N2/O2/H2),
Detector(FID). The system was covered with aluminum foil which served as light
reflector.
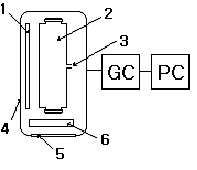
Fig.
2. Schematic diagram of the experimental set-up: 1. UV lamp; 2. photoreactor;
3.
sampling port; 4. foiled chamber; 5. cooling fan; 6. thermocontroller.
2.6. Photodegradation of IPA
under several coating conditions on POF
The POF of 30cm units was soaked in acetone for 90 sec, pulled it
out and washed with distilled water. TiO2 slurry solution consisted
of TiO2, binder and ethanol. The weight ratio of TiO2/binder/ethanol
was 0.05/1/5.6, respectively. We used several binders such as inorganic
binder(KR-400), organic binder(A-9540) and inorganic∙organic
hybrid(GPTMS∙TMOS) resins. Furthermore, the ratio of TMOS : TiO2
was from 0.05 to 1. This slurry solution was treated by ball mill at 300 rpm
for 2hr. Pretreated POF were dipped into the stirred 15wt% TiO2
slurry solution and then oven-dried at 80℃ for 2hr. TiO2-coated
POF were moved to reactor. The inner temperature of reactor was maintained at
25℃. IPA 430ppm was injected in reactor. When thermal and diffusion
state is stable. then, UV light source was turned on. Photodegradating sample
was transferred to a GC by syringe in 0.5ml scale per few minute intervals. The
present study identified the target IPA by retention times using GC/FID
analysis.
3. RESULTS AND DISCUSSION
3.1. Influence of the amount
of TiO2
The preliminary processes were examined on the surface of
PMMA(polymethyl methacrylate) instead of POF, in order to identify the
influence of the amount of TiO2 on the photodegradation of IPA. As
seen in Fig. 3, the efficiency of the photodegradation of IPA was proportional
to the amount of TiO2(0.01g, 0.02, 0.03g).
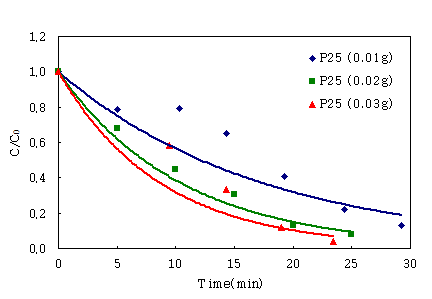
Fig. 3. The influence of the amount of TiO2(0.01g,
0.02, 0.03g) on the photodegradation of IPA.
3.2. Effect of the IPA
concentration
Fig. 4 shown the effect of the IPA concentrations (215, 430,
1000ppm) using TiO2 0.02g as the previous results. For all three IPA
concentrations, the tendency of IPA degradation showed the similar
pattern. The photodegradation efficiencies were about 80% for 215ppm at
retention time 9.5 min, nearly 100% for 1000ppm at retention time 24min.
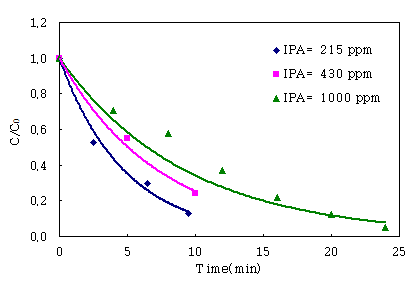
Fig.
4. The effect of the IPA concentrations (215, 430, 1000ppm) on TiO2
0.02g.
3.3. Effect of diluting
solvents on TiO2 dispersion
Degradation profiles were shown in Fig. 5 with data for several
solvents such as ethanol, tert-butyl alcohol, 1-octanol, from low to
high-chained alcohols. The photodegradation efficiency was essentially
dependent of the diluting solvents used in the coating processes. As shown,
Ethanol exhibited higher performance than other solvents, 1-octanol was lower
performance. From results, the effect of IPA photodegradation decreased with an
increase in the carbon chain number.
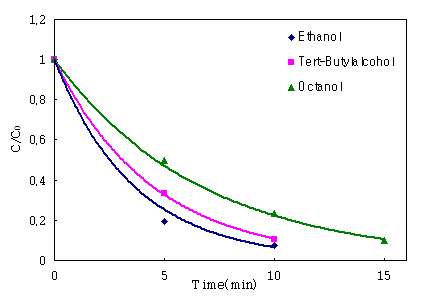
Fig.
5. Effect of diluting solvents on TiO2 dispersion
3.4. Effect of binders
As the previous results, we have used ethanol as
diluting solvent. The dependence of binders on the photodegradation of IPA has
been studied using binders such as Inorganic(KR-400), organic(A-9540) and
inorganic∙organic hybrid(GPTMS, TMOS) resin. The weight % of TiO2(P-25)
contains 15wt%. Fig. 6 shows the results of binders to the same 15wt% P-25 and
ethanol on POF. From the figure, photodegadation activities of IPA on POF
decreased on the binder GPTMS than other binders. Organic binder(A-9540)
showed the highest activity for IPA degradation. It is known that this
activities difference decrease with increasing carbon-chain length. This reason
is similar well-known original photocatalytic phenomenon, which leads to the
breakdown of organics[11], Deduced other reasons is UV reflection and UV
adsorption of binder.
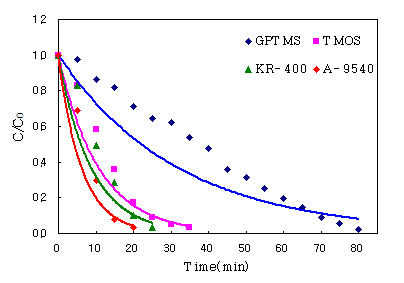
Fig. 6. The dependence of binders on 15wt% P-25 and
ethanol.
3.5. Effect of the amount of TMOS
Organic binders was decomposed by photocatalyst, inorganic binders
were lower adhesive than organic binders. Then, we have adopted the use
of inorganic∙organic hybrid(GPTMS, TMOS) resins as binder, ethanol as
diluting solvent, 15wt% P-25 as TiO2. The ratio of TMOS to P-25 was
0.05∼0.1. The Comparatively activity of
the amount of TMOS can be seen from Fig. 7. Increased amounts of TMOS decreased
the activity of TiO2-coated POF. The amount of TMOS was less , the
activity was more. As shown in figure, the zero amount of TMOS were the most
active. From these results, the least amount of TMOS as binder in
the coating conditions on POF was needed to exhibit an effective
photodegradation of IPA.
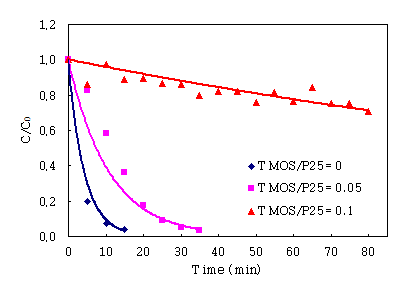
Fig.
7. Comparison of IPA photodegradation activities with difference
TMOS/P-25= 0; 0.05; 0.1.
4. CONCLUSIONS
This study showed that the photodegradation of IPA on POF were
examined under TiO2-coating condtions. The photodegradation
efficiency of IPA decreased with an increase in the carbon chain number.
Ethanol exhibited higher performance than other solvents. Organic
binder(A-9540) among inorganic(KR-400), organic(A-9540) and
inorganic∙organic hybrid(GPTMS, TMOS) resins as binders showed the
highest activity for IPA degradation. we have adopted the use of
inorganic∙organic hybrid(GPTMS, TMOS) resins as binder. The ratio of TMOS
to P-25 was 0.05∼0.1. Increased
amounts of TMOS decreased the activity of TiO2-coated POF. The least
amount of TMOS as binder in the coating conditions on POF was needed to exhibit
an effective photodegradation of IPA.
REFERENCES
1. T. Hisanaga, K. Harada, K. Tanaka, J. Photochem.
Photobiol. A: Chem., 54, 113(1990).
2. A. Bouzaza, A. Laplanche, J. Photochem. Photobiol. A:
Chem., 150, 207(2002).
3. A. Mills, R. H. Davies, D. Worsley, Chem. Soc. Rev., 417(1993).
4. F. W. Wilkins, D. M. Blake, Chem.
5. H. Yammashita, M. Harada,
J. Misaka, M. Takeuchi, K. Ikeue, M. Anpo, J. Photochem.
Photobiol.A:
Chem., 148, 257(2002).
6. A. J. Bard, J. Phys. Chem., 86, 172(1982).
7. G. Hodes, M. Gratzel, Nouv. J. Chem., 8, 509(1984).
8. R. Sun, A. Nakajima,
136, 111(2000).
9. Y. Ohko, A. Fujishima, K. Hashimoto, J. Phys. Chem., B 102,
1724(1998).
10. J. Péral, D. F. Ollis, J. Catal., 136, 554(1992).
[ BWW Society Home Page ]
© 2005 The BWW Society/The Institute for the Advancement of Positive Global Solutions