Science: Materials & Technology:
B2O3 in Magnesium Oxide from Seawater
Dr. Vanja Martinac, Dr. Nedjeljka Petric, Dr. Miroslav Labor and Dr. Vedran Tripalo
Faculty of Chemical Technology, University of Split, Croatia
ABSTRACT
The effect of pH of the rinsing agent on the
content of B2O3, CaO and MgO in calcined magnesium oxide
obtained from seawater by substoichiometric precipitation (where precipitation
of magnesium hydroxide took place with 80% of the stoichiometric quantity of
the dolomite lime as the precipitation agent) has been investigated.
The purpose of the study has been to ensure
high purity magnesium oxide, particularly regarding the B2O3
content, because boron causes a problem in refractory magnesia for specialized
refractory applications where high hot-strength is required.
It has been established that the content of B2O3
in magnesium oxide samples is significantly reduced when the pH value of the
agent used for rinsing the magnesium hydroxide precipitate increases.
Keywords:
Magnesium Oxide, Seawater, B2O3 content,
Substoichiometric Precipitation, Rinsing Agent
1.
INTRODUCTION
Although thermal decomposition of magnesite
(MgCO3) is the most frequent method of obtaining magnesium oxide [1-5], reserves of quality magnesite have been
decreasing, so that chemical procedures for obtaining magnesium oxide [6-16], especially high purity one, have been studied
all over the world for a number of years. One of these procedures is obtaining
magnesium oxide from seawater.
Magnesium oxide obtained from seawater [9-16] is one of the most important materials used
for production of high temperature resistant ceramic, and its advantage does
not lie only in large reserves of seawater (1 m3 contains 0.945 kg
of magnesium) but in higher purity of magnesium oxide sintered (above 98 mass
%).
The basis of this technology is obtaining
non-soluble magnesium hydroxide from seawater by precipitation with dolomite
lime or lime from limestone, and by settling of the precipitate formed. The
precipitate is washed and calcined to form caustic magnesia (MgO).
The settling rate is the "bottleneck"
of this technology and it is one of the most important unit operations
determining the cost of production. The settling rate can be significantly
increased if small quantities of organic long chain polymers [15-18] are used, as well as if incomplete
precipitation of magnesium hydroxide takes place. At precipitation of 80%, the
capacity of the thickener increases by 86.5 % relative to complete
precipitation [15].
In such a case, i.e. when this precipitation
method is applied, the boron content adsorbed onto magnesium hydroxide during
the precipitation process is somewhat higher than at stoichiometric
precipitation, and should therefore be reduced. Boron is a particularly
problematic impurity for magnesia to be used as high-quality refractory
material. Thus, boron can be a problem in refractory magnesia for specialized
refractory applications where high hot-strength is required.
The aim of this study was to examine the method
of rinsing the precipitate of magnesium hydroxide obtained from seawater by
substoichiometric 80 % precipitation in order to reduce the boron (B2O3)
content in the final product, i.e. magnesium oxide.
2.
EXPERIMENTAL
The seawater used for precipitation of magnesium
hydroxide had the following content of magnesium oxide and calcium oxide:
2.1794 g dm-3 MgO; 0.5557 g dm-3 CaO.
The composition of the dolomite lime used was
as follows (mass %): MgO = 40.90 %, CaO = 57.89 %, SiO2 = 0.102 %,
Fe2O3 = 0.319 % and Al2O3 = 0.866
%.
The seawater has first been pretreated to
remove the bicarbonate and carbonate ions by adding a defined quantity of H2SO4
with on-line control through pH measurement (pH = 3.8 - 4.0), and degassing the
acidified water to remove the released CO2. Degassing was
accomplished in a desorption tower by blowing air. The flow rate of the induced
air was 120 dm3 h-1, and the volumetric flow rate of
seawater through the desorption tower was 6 dm3 h-1.
After pretreatment of seawater, precipitation of magnesium hydroxide took place with 80% of the stoichiometric quantity of dolomite lime. The precipitation reaction took 30 min., a magnetic stirrer was used. The sedimentation rate was increased by the addition of the Flocal-B (polyacrilamid) flocculant, in the optimum quantity. The experimental procedure to determine the optimum quantity of Flocal-B has been described in a previous investigation [15].
The precipitate obtained was then decanted and
rinsed. The operating conditions were varied depending on the agent used for
rinsing the magnesium hydroxide precipitate.
The rinsing agent was:
- distilled water, pH =
5.95
-
alkalized
distilled water, pH = 11.00 and pH = 12.50, which was alkalized by addition
of concentrated NaOH.
The magnesium hydroxide precipitate obtained
was rinsed by decanting and filtering. The rinsing and decanting procedure was
repeated five times with approximately 1 dm3 of the rinsing agent.
Then the magnesium hydroxide precipitate was filtered through a number of
funnels. The rinsing agent used with magnesium hydroxide precipitate on the
filter paper was the same as the one used for rinsing by decanting. The
procedure was repeated five times, i.e. until rinsing was completed.
The magnesium hydroxide obtained was dried at
105oC, and then calcined at 950oC for 5 h to form caustic
magnesia. The boron content in the sample examined was determined
potentiometrically. The variation coefficient for the method applied is ± 1% [19].
The results listed represent average values of
a series of measurements. (An average of five analyses in each case.)
3.
RESULTS AND DISCUSSION
Examinations were carried out with magnesium
oxide samples prepared by rinsing the precipitate with alkalized distilled
water, non-alkalized distilled water and without previous rinsing of the
magnesium hydroxide precipitate.
Table 1 shows operating conditions during
rinsing of the magnesium hydroxide precipitate obtained from seawater by
substoichiometric 80 % precipitation, as well as the experimentally obtained
values for the composition of magnesium oxide samples.
Table 1. Chemical compositions (mass %) of
magnesium oxide samples (80% precipitation)
after calcining at 950 oC / 5 h.
|
No. of samples |
Rinsing agent |
pH of rinsing agent |
CaO |
MgO |
B |
B2O3 |
|
mass % |
||||||
|
1. |
distilled water |
5.95 |
0.85 |
98.62 |
0.0548 |
0.1764 |
|
2. |
alkalized distilled water |
11.00 |
0.95 |
97.89 |
0.0372 |
0.1198 |
|
3. |
alkalized distilled water |
12.50 |
1.28 |
97.83 |
0.0161 |
0.0518 |
|
4. |
without rinsing |
|
2.613 |
96.77 |
0.0602 |
0.1937 |
The experimental part of this study has
examined the effect of the rinsing agent used for rinsing the precipitate of
magnesium hydroxide obtained from seawater by stoichiometric 80 %
precipitation. Examinations were carried out in order to reduce the boron (B2O3)
content in the final product – magnesium oxide, because the hot-strength
properties of certain magnesia refractory product are significantly affected by
their boron content.
Boron occurs in seawater partly as
non-dissociated orthoborate acid (H3BO3) and partly as
borate ions (H2BO3-), and during the magnesia
precipitation process boron is adsorbed onto magnesia. The orthoborate acid is
a weak acid with the following dissociation constants:
|
H3BO3 = H+ +
H2BO3- |
K1 = 5.8.10-10 |
|
H2BO3- = H+
+ HBO32- |
K2 = 1.8.10-13 |
|
HBO32- = H+
+ BO33- |
K3 = 1.6.10-14 |
The concentration of higher oxidation level
ions HBO32- and BO33- is very low.
By calculating the dissociation rate, one can establish the molal
concentrations of H2BO3-, HBO32-
and BO33-, as well as the molal dissociation rate for
every degree of dissociation of the orthoborate acid.
Fig. 1. shows concentrations of H2BO3-,
HBO32- and BO33- relative to pH of
the rinsing solution (5.95, 11.00 and 12.50), as well as to pH of seawater
during the precipitation process (9.6), while figure 2 shows the molal
dissociation rate of H3BO3 relative to pH.
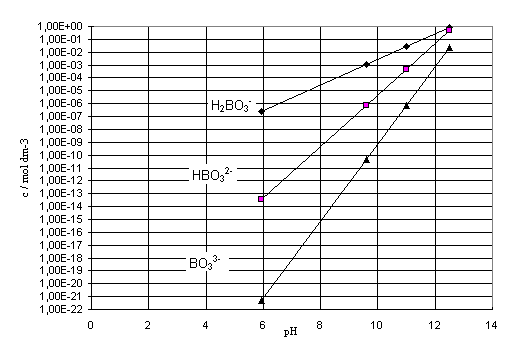
Figure 1. The concentration of H2BO3-,
HBO32- and BO33- relative to pH
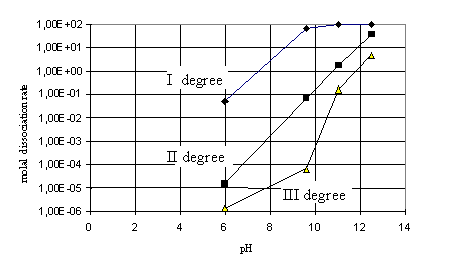
Figure 2. The molal dissociation rate of the
orthoborate acid (H3BO3) relative to pH
For 80% precipitation of magnesium hydroxide
from seawater by dolomite lime, the pH value is 9.6 during reaction
precipitation and settling of the precipitate formed. In that case the
orthoborate acid dissociation in the first degree is 69.78 %, which contributes
to a significant increase of the B2O3 content in the
product, i.e. in magnesium oxide obtained from seawater (0.1937 mass %).
Experimental data for chemical composition of
magnesium oxide samples used indicate that the method of rinsing of magnesium
hydroxide precipitate greatly affects the chemical composition of these
samples, primarily as regards the content of CaO and B2O3 in
calcined magnesium oxide. Examinations show that when the magnesium hydroxide
precipitate is rinsed with alkalized distilled water with pH = 12.50, the B2O3
content in the final product is greatly reduced.
Experimental
results indicate that the MgO content in the sample prepared by rinsing the
magnesium hydroxide precipitate with distilled water with pH = 5.95 amounts to
98.62 mass %, being slightly higher than the MgO content in the sample prepared
by rinsing the magnesium hydroxide precipitate with alkalized distilled water
with pH = 11.00 (MgO = 97.89 mass %), as well as with pH = 12.50 (MgO = 97.83
mass %). They differ for 0.8 %. In the magnesium oxide sample prepared without
rinsing the magnesium hydroxide precipitate, the MgO content is markedly lower
(96.77 mass %) due to higher impurity content.
Although
examinations indicate that the sample contains a significant CaO content (0.95 mass %, and 1.28 mass %, respectively)
it is lower by half than in the sample prepared without rinsing the magnesium
hydroxide precipitate (CaO = 2.613 mass %).
As for the B2O3 content,
Table 1 indicates that rinsing with alkalized distilled water of a high pH
value (11.00 and especially 12.50) significantly reduces the quantity of boron
adsorbed. When alkalized distilled water with pH = 11.00 is used to rinse the
magnesium hydroxide precipitate, the B2O3 content in the
final product – magnesium oxide- amounts to 0.1198 mass %, which is 38.15 %
less than the B2O3 content in the sample prepared without
rinsing of the magnesium hydroxide precipitate (B2O3 =
0.1937 mass %). If alkalized distilled water with pH = 12.50 is used to rinse
the magnesium hydroxide precipitate, the B2O3 content is
0.0518 mass % which is 73.26 % less than the B2O3 content
in the sample prepared without rinsing the magnesium hydroxide precipitate.
Therefore, we can conclude that the B2O3
content is significantly lower in the sample prepared with the rinsing agent
with high pH values, especially pH = 12.50.
The increase in the pH value of the rinsing
agent evidently reduces the content of boron adsorbed onto the magnesium
hydroxide precipitate.
The increase in the pH value of the rinsing
agent acts in direction of fuller dissociation of orthoborate acid, i.e.
increased concentration of ions of higher dissociation degree, which are
adsorbed more strongly. Graphical representations in Fig.1. and Fig.2. indicate
that at pH = 12.50 the H2BO3- concentration is
0.85 mol dm-3, the HBO32- concentration is
0.49 mol dm-3, and the BO33- concentration is
2.45.10-2; namely, H3BO3 has
dissociated to 99.94 %, H2BO3- to 36.27 %, and
HBO32- to only 4.82 %.
Thus, at pH = 12.50 dissociation in the first
degree is complete, while only a third of the second degree dissociation has
taken place, and the B2O3 concentration is very low. If
the process is conducted like this, there is the possibility of adsorption of
ionic boron forms, but it does not take place. Namely, the highly alkaline
medium (pH = 11.00, and especially pH = 12.50) in rinsing the magnesium
hydroxide precipitate by alkalized distilled water primarily affects the
preferred adsorption of OH- ions. So, due to alkaline medium,
adsorption of ionic forms of boron is significantly lower. However, increased
pH affects increased adsorption of Ca2+ ions on the Mg(OH)2
precipitate in a strongly alkaline medium,
i.e. at pH 12-13, but not to a greater degree.
4. CONCLUSION
- It has been established that the increase in
the pH value of the rinsing agent reduces the content of boron (B2O3)
adsorbed onto the precipitate of magnesium hydroxide from seawater
- Magnesium oxide samples obtained by rinsing
the magnesium hydroxide precipitate with alkalized distilled water, pH = 12.50,
contain approx. 70% B2O3 less than the samples prepared
by rinsing the magnesium hydroxide precipitate with non-alkalized distilled
water.
- The increase in the pH value of the rinsing
agent increases the mass % of CaO, but not to a greater degree
REFERENCES
1.
M. Chaudhuri, A.
Kumar, A. K. Bhadra, G. Banerjee, Interceram., 39, 26 (1990).
2.
M. N. Chaudhuri,
A. Kumar, A. K. Bhadra, G. Banerjee, L. S. Shondeep, Ceramic Bull., 71, 345 (1992).
3.
M. A. Serry, M.
A. Mandour, A. G. M. Osman, L. G. Girgs, Interceram., 45, 162 (1996).
4.
M. A. Serry, H.
S. Abd Elwahab, A. A. Abd Allah, Sil. Ind., 64, 129 (1999).
5.
L. Nan, C.
Shi-Hua, Z. Dao-Yun, Sci. Sinter., 19,
31 (1987).
6.
J. Kozač,
Mineralia Slovaca, 29, 80 (1997).
7.
M. Turek, W.
Gnot, Ind. Eng. Chem. Res., 34, 244
(1995).
8.
M.
Ćosić, B. Pavlovski, E. Tkalčec, Sci. Sinter., 21, 161 (1989).
9.
H. Tsuge, Y.
Kotaki, S. Asano, 7th Symposium on Salt, Elsevier Science Publishers B. V., Amsterdam,
Vol. II, 219 (1993).
10.
M. J.
Gildersleeve, R. J. Brook, Br. Ceram. Trans., 83, 181 (1984).
11.
C. Sims, Industr.
Minerals, 7, 21 (1997).
12.
W. C. Gilpin, N.
Heasman, Chem. Ind., 16, 567 (1977).
13.
N. Heasman, Gas
Wärme International, 28, 329 (1979).
14.
J. C. Hicks, S.
Tangney, Ceram. Bull., 59, 711
(1980).
15.
B. Petric, N.
Petric, Ind. Eng. Chem. Process Des. Dev., 19,
329 (1980).
16.
D. Barba, V.
Brandani, G. Di Giacomo, P. U. Foscolo, Desalination, 33, 241 (1980).
17.
R. N. Vohra, K.
N. Patel, B. K. Shukla, Chem. Age of India, 19, 441 (1968).
18.
N. Petric, V.
Martinac, M. Labor, O. Jurin, KZLTET, 33,
473 (1999).
19.
F. Culkin, The
major constituents of sea water, in: Chemical Oceanography, Eds. J. P. Riley,
G. Skirrow, Vol. 1, Academic Press, London, 1975.
[ BWW Society Home Page ]
© 2003 The BWW Society/The Institute for the Advancement of Positive Global Solutions