Kinetics
for the Formation of YBa2Cu3Oy Superconductors
from the Oxide Precursor Containing BaCuO2.5
by
Dr. Hai-woong Park and Dr. Jin-Wook Ha*
Department of Advanced
Materials
*Department of Environmental
Abstract
The oxide precursor containing BaCuO2.5, Y2O3
and CuO with overall composition of Y: Ba: Cu: O= 1: 2: 3: 7.4 was synthesized
from metallic Y, metallic Cu and Ba(NO3)2 at 600℃. The precursor was
successfully converted to the superconducting Y123 orthorhombic phase with heat
treatment at 900~1020℃ in
air without an additional oxygenation process at low temperature. For the
precursor powder, the orthorhombic Y123 superconducting phase was directly
formed within 10 minutes. In the case of the 1mm thick silver-sheathed sample,
Y123 superconducting phase was obtained after soaking for 30 minutes. at 950℃ and cooled at 100℃/h in air. In the melt
processing of the pellet samples at 1020℃, the superconducting phase was formed
within 30 minutes. The directly formed superconducting phases at high
temperature from both the silver-sheathed and the pellets showed 92K of the
critical temperature.
1. INTRODUCTION
In the synthesis of YBa2Cu3Oy(Y123)
superconductor, many researchers have used Y2O3-BaO-CuO
system as a starting materials. The carbonate(BaCO3) in the system is
difficult to decompose even at high temperature and such high temperature
easily result in particle coarsening before sintering[1, 2]. Studies of this
system[3] also indicated that the value of y in the YBa2Cu3Oy
varied with oxygen partial pressure and temperature. The oxygen solubility in
Y123 vary continuously for the composition range corresponding to values of y
of about 6.3-7.5. Because of such phase equilibrium and relatively low
temperature during the oxygenation process, the probability of forming a
non-superconducting phase is very high.
From the conventional processes, the end product is a
tetragonal YBa2Cu3Oy, y=6.3~6.6 which need to
be oxygenated to the orthorhombic YBa2Cu3Oy, y≈7.0.
Since the rate of oxygen diffusion through Y123 is very slow and the tendency
of the sample to form microcracks during oxygenation process limit
superconducting properties and sample sizes[4, 5].
In the previous studies[6, 7], a two stage processing was
successfully applied for the synthesis of bulk Y123 superconductors via the
intermediated precursor(IP). The intermediate precursor was produced by
attrition milling of suitable starting materials and subsequent heat treatment
in air. The attrition milling generate finer powder and reduce milling time
compared to conventional milling. During attrition milling, starting powders
were refined to submicron scale through repeated cold welding and fracture, and
they were converted to BaCuO2.5, Y2O3 and CuO in
a subsequent heat treatment. The
intermediate precursor contained the overall composition of Y: Ba: Cu: O = 1: 2:
3: Z, Z>7.0 where the oxygen content of the intermediated precursor was
higher than that of the orthorhombic Y123. In both the thermomechanical processing
of the bulk sample at 900~950℃
and the melt processing at 1020℃,
the intermediate precursor were utilized for the formation of bulk Y123
superconductors through the direct participation of BaCuO2.5. In
this approach, orthorhombic Y123 was directly obtained without additional
oxygenation process and thus the difficulty of an additional oxygenation
process in dense bulk samples can be avoided.
The objective of this work is to study the appropriate
thermodynamic and kinetic conditions in both silver-sheathed and bulk Y123
samples for the direct formation of orthorhombic Y123 at 950~1020℃ in ambient air from the
oxide precursor.
2.
EXPERIMENTAL PROCEDURE
In this study, Y123 superconducting phase was synthesized
in two stage processes. In the first stage, an intermediate precursor was
synthesized via high energy milling and subsequent heat treatment from the
starting powders. In the second stage, the IP was converted into high
temperature superconductors through thermomechanical processing or heat
treatment.
2.1
Synthesis of the IP
The high purity powders of metallic yttrium, metallic
copper and barium nitrate were used as starting chemicals. The starting powders
were milled for 20~65 hours in an attritor. Then the composite powder was heat
treated at 600℃ in
an argon environment. The milled powder was carefully heated to 600℃ under a continuous stream
of high purity argon gas. After 5 hours of holding at the peak temperature, the
powder was cooled to room temperature.
2.2
Heat treatment of the IP precursor powder
To determine the nature of the reactions and intermediate
phases appearing during the formation of the Y123 compound, a series of rapid
heat, soak, and quench experiments for the powder precursor were conducted at
selected temperatures. In these series of experiments, 0.5g of precursor was
introduced into a furnace preheated at 10~15℃ above of each endothermic peaks in DTA
and then soaked for 1~300 minutes in air.
2.3
Thermomechanical processing of silver-sheathed sample
The IP powder was packed inside the silver tube by
powder-in-tube techniques or cold pressed into pellet form. The silver-sheathed
samples were deformed mechanically by repeated cold swagging or hot rolling.
The packed tube was cold swagged for 75%. After swagging, the tube was cut to
short pieces and sealed by a die forge. The samples were placed in a preheated
furnace chamber at 950℃.
The samples were rolled repeatedly until the final thickness of the sample
reached to the predetermined value. After the thermomechanical processing,
various types of heat treatment experiment were conducted where the peak
temperatures, soaking time, and cooling rates were varied within the range of 900~950℃. At 900℃, the rolled samples were
held for 30~90 minutes by 15℃/h
to 900℃
and by 100℃/h
to room temperature. The samples were also held for 30 minutes at 950℃ and the cooling rate was
varied.
2.4
Pellet sample
The weighed precursor was formed into 0.64cm diameter and
0.1cm thickness by 300MPa uniaxial pressure. The green density of the formed
powder was obtained by geometrical condition. The density of the pellets were
about 4.2g/㎤.
After forming the IP into pellet form, various types of rapid heat, soak and
cooling experiments were conducted at 1020℃. Through these experiments, reaction
time for the formation of Y123 was determined. The samples were also held for
30~60 minutes at 1020℃
and the cooling rate of the pellets were varied from air quenching to 5℃/h. The environment of the
furnace was controlled by flowing air during heat treatment.
2.5
Characterization
The powder samples were analyzed with various techniques.
The chemical compositions or phase evolution of the samples were analyzed by
XRD. TGA experiment was performed to measure the weight change of the powder IP
or bulk samples during heating process. For the powder IP, the powder was
loaded into a platinum boat at room temperature. The sample was heated to 950℃ at 5 or 50℃/min in air flow. The
weight loss of powder was measured with respect to time. For bulk samples, a
1mm thick and 2.5mm diameter sample at room temperature was inserted into the
TGA equipment which was preheated to 950℃.
3. RESULTS AND DISCUSSION
In the previous works[6, 7], we successfully synthesized the
IP consisting of Y2O3, BaCuO2.5 and CuO from
the metallic Y, metallic Cu and Ba(NO3)2 via high energy
attrition milling and appropriate heat treatment at 600℃. The formed precursor
contains higher oxygen content than that of orthorhombic Y123 superconducting
phase which requires oxygen content of 6.8~7.0. The IP powders were directly
converted to orthorhombic Y123 phase at high temperature without an additional
oxygenation process at low temperature. These results were due to the slow
decomposition of BaCuO2.5 at a fast heating rate. During the
decomposition of BaCuO2.5 to BaCuO2, the BaCuO2.5
phase release its excess oxygen and result in formation of orthorhombic Y123
superconducting phase.
3.1 Kinetic for the formation of orthorhombic Y123
Orthorhombic Y123 phase is not stable at high temperature[8].
Kishio et al.[9] showed that the oxygen content of Y123 at high temperature
decreased as a function of time due to oxygen diffusion. As shown in figure 1,
orthorhombic Y123 phase formed in early stage of heat treatment at 900~1020℃ transformed to tetragonal
Y123 when the soaking time increased to 300 minutes. Therefore, it is necessary
to reduce the temperature after the conversion of the precursor to orthorhombic
Y123 phase. In order to determine kinetic conditions for the formation of
orthorhombic Y123, we conducted two different series of experiments. First, we
determined the minimum reaction times at 900~1020℃ through a series of rapid heat, soak,
and quench experiments in air environment. Second, we performed a TGA
experiment for a bulk sample in air to calculate the oxygen content of the bulk
sample. In the TGA study, the bulk sample was rapidly heated to 950℃.
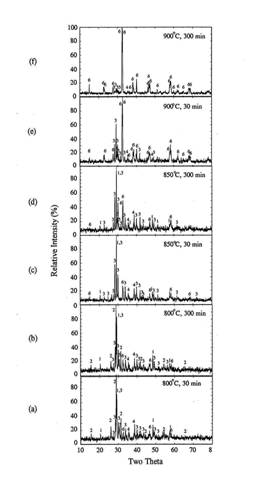
Figure
1. XRD
patterns of heat treated IP in rapid heat, soak and quench experiments.
3.1.1
Minimum reaction time at 950-954℃
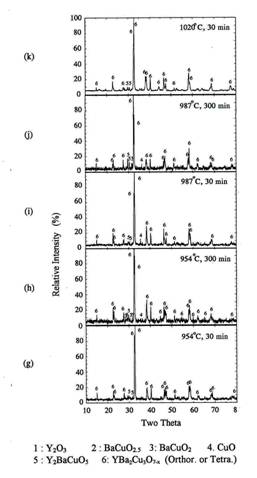
Figure 2 show the XRD patterns for the powder
precursors(0.5g) which were rapidly heated at 954℃, soaked for 5, 10, or 30 minutes and
quenched to room temperature.
Orthorhombic Y123 phase was the main peak after 5 minutes
holding at 954℃.
Some amount of BaCuO2 and CuO peaks were also detected. When the
soaking time increased to 10 minutes, only orthorhombic Y123 phase and traces
of 211 were obtained. The XRD pattern after 30 minutes also shows orthorhombic
Y123 and traces of 211. We think that the small amount of 211 phase detected in
figure 2(b) and (c) was formed by the peritectic reaction between Y123 and
CuO because it was not formed until 5 minutes holding at 954℃[10]. Therefore, the
reaction for the formation of the orthorhombic Y123 powder IP was completed
within 10 minutes at 954℃.
Figure
2. XRD of
the powder IP after rapid heat, soak and quench at 954℃ in air.
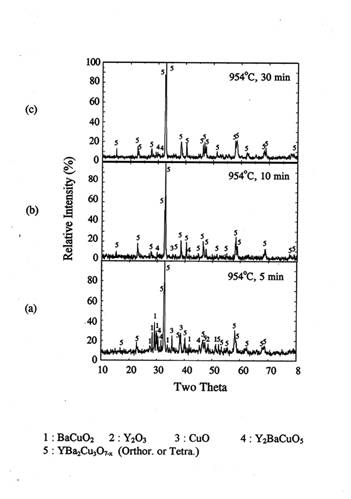
The pellet samples(P1) were rapidly heated to 950℃ and held for 2, 5, 10, and
30 minutes and subsequently quenched in air. Figure 3 shows the XRD patterns of
the quenched bulk samples.
During early stages of reaction(2 min, figure 3(a)),
BaCuO2.5 was present along with the reaction product Y123 and the
reactants Y2O3 and CuO. An appreciable amount of BaCuO2
was also present. As the reaction progressed for 5 minutes(figure 3(b)), BaCuO2.5
disappeared completely and the peaks of BaCuO2 also decreased
drastically. Orthorhombic Y123 peak became predominant. After 10 minutes of
reaction, only orthorhombic Y123 with traces of 211 phases were observed. After
30 minutes holding, XRD pattern shows no difference with that in the case of 10
minutes holding case. Therefore, the formation of orthorhombic Y123 phase was
completed within 10 minutes.

Figure 3. XRD of the
pellet IP after rapid heat, soak and quench at 950℃ in air.
The above results suggest the minimum reaction time for
formation of orthorhombic Y123 phase at 950~954℃ was less than 10 minutes for both
powder and bulk samples and the reaction time was not affected by the sample
geometry in present condition. Ten minutes of reaction time at 950~954℃ is shorter than that of
the decomposition of BaCuO2.5 via equation 1.
BaCuO2.5
→ BaCuO2 + 0.5O2 [Eq. 1]
This is because in the TGA experiments at 50℃/min of heating rate shown
in figure 4, the sample was heated from room temperature to 950℃ in about 19 minutes and
during this period BaCuO2.5 did not decompose completely. Thus, at
950℃ or
higher temperature, the reaction kinetics for the formation of orthorhombic
Y123 from the IP is faster than the decomposition of BaCuO2.5 via
equation 1. In such a case, the undecomposed BaCuO2.5 can
participate in the formation of orthorhombic Y123.
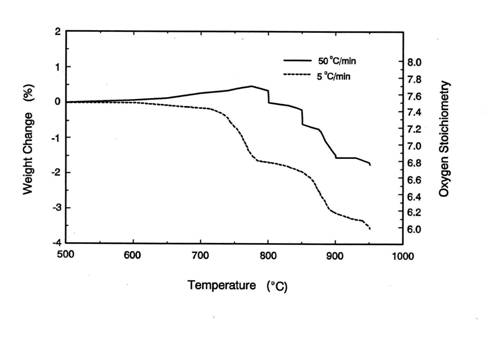
Figure 4. The weight loss of the IP at different heating
rates in TGA experiments.
3.1.2
Weight loss of the bulk sample at 950℃
To estimate the decomposition period of orthorhombic Y123
phase, a TGA experiment for a pellet sample was conducted at 950℃. The dimension of the
pellet sample was 1㎜ in
thickness and 2.5㎜ in
diameter. Figure 5 shows the weight loss of the pellet with respect to time. In
this Figure, the Y: Ba: Cu: O molar ratio was also noted in the right Y axis.
From the weight loss of the sample, we calculated the oxygen content of the
sample.
The total weight loss of the sample after reaching to
steady state was about 3.5%. Since the initial oxygen content was 7.5, the
final oxygen content will be about 6.0. The total relaxation time of the sample
from 7.5 to 6.0 was about 58 minutes. The points P and B(10 min) in the time
axis represent the periods when the formation of orthorhombic Y123 from the
powder and bulk precursor were respectively completed.
The point D, where Y: Ba: Cu: O = 1: 2: 3: 6.5, correspond to the complete
decomposition of BaCuO2.5 to BaCuO2 via equation 1. The
point D is also consistent with an approximate transition temperature of
orthorhombic Y123 to tetragonal Y123 phase.
For the present conditions, the minimum reaction time for
the formation of orthorhombic Y123 in both powder(P) and bulk samples(B) were
far shorter than the time needed for complete decomposition of BaCuO2.5.
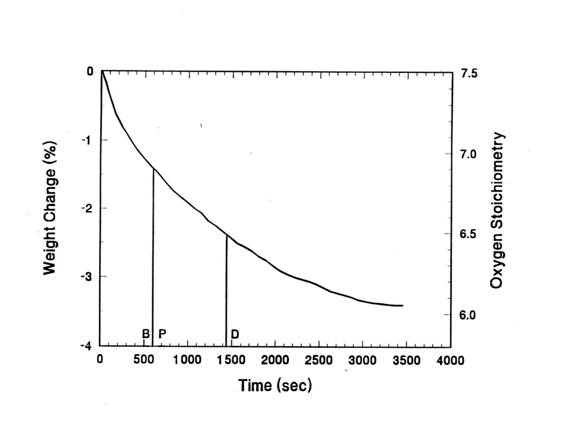
Figure
5. The weight loss of the pellet IP at 950℃ in air. P and B correspond to minimum time for the formation of orthorhombic Y123 in air
from powder and bulk IP. D represents the
composition
corresponding
to complete decomposition of BaCuO2.5.
In this situation, the undecomposed BaCuO2.5 can
participate to the formation of orthorhombic Y123 by equation 1. This result is
consistent with the observation of BaCuO2.5 in early stage of
heat treatment experiment of the bulk sample at 950℃ shown in figure 1. The TGA
result also suggests that the released oxygen from the partial decomposition of
BaCuO2.5 to BaCuO2 via equation 1 can be
retained inside the sample because the oxygen content at P and B is higher than
6.5. In this case, the retained oxygen will in-diffuse to tetragonal phase
during cooling process.
Umemura et al.[11] measured the weight loss in a dense
Y123 sintered sample (bulk density ≈91%) at 300~950℃. They estimated the
temperature dependence of the out-diffusion constant as Dout =3.7×10-5
exp(-0.50 ℮V/kBT). Tu et al.[12] also determined the
diffusivity of oxygen in orthorhombic polycrystalline in Y123(x=0) as D=0.035
exp(-1.3 ℮V/kT) [㎠/sec].
We calculated the estimated oxygen diffusion time of the 1㎜ thick bulk sample at 950℃ from Umemura and Tu's
results. The calculated oxygen diffusion times were about 35 and 69 minutes,
respectively. Even though our TGA result shows some discrepancies with
Umemura's value, it was very close with Tu's calculated relaxation time. The
result of TGA experiment for bulk sample at 950℃ suggests that the orthorhombic Y123
phase can be obtained without and additional oxygenation process if the
reaction time for a 1㎜
thick bulk sample is less than about 25 minutes. This TGA result is consistent
with the results of reaction time experiments.
3.2
Effects of cooling rate at 950℃
In this series of experiments, we prepared
silver-sheathed samples from the same batch of IP. We fixed all the
experimental conditions except cooling rate. The fixed experimental conditions
are shown in table 1.
Table 1. Experimental conditions for the silver-sheathed
samples
|
Parameters |
Specifications |
|
Milling time |
20h |
|
Heating rate(1st H.T.)a |
50℃/h |
|
Peak temperature(1st H.T.) |
600℃ |
|
Holing time(1st H.T.) |
5h |
|
Sample thickness |
3.2㎜(after swagging) |
|
|
1.0㎜(after hot rolling) |
|
Heating rate(2nd H.T.)b |
rapid heat |
|
Peak temperature(2nd H.T.) |
950℃ |
|
Holing time(2nd H.T.) |
30minutes |
a Heating rate for the formation of the
IP from the milled precursor.
b Heating rate for the formation of Y123
phase from the IP.
The silver-sheathed samples having 1 ㎜ in
thickness were rapidly heated to 950℃, soaked for 30 minutes and
subsequently cooled in purified air at different cooling rates. The cooling
rates were air quenching, 200℃/h,
and 100℃/h,
respectively. Figure 6 shows the XRD patterns of the cooled samples. All
samples show orthorhombic Y123 phase as observed by the relative intensities at
2ɵ=32.5~32.8° and
46.6~47.5°.
When the cooling rate decreased from air quenching to 200
or 100℃/h
in air, the relative intensities of orthorhombic Y123 peaks at (013)~(011)
doublet reflections between 2ɵ=32.5
and 32.8℃
showed larger separation. At 100℃/h cooling rate, 2ɵ values for (013) and (011) peaks were
32.7 and 32.9°, respectively. Based on the reference XRD data[13], these
2θ values match with those in YBa2Cu3O7 case.
This means that the total oxygen content of the sample is close to 7.0[13].
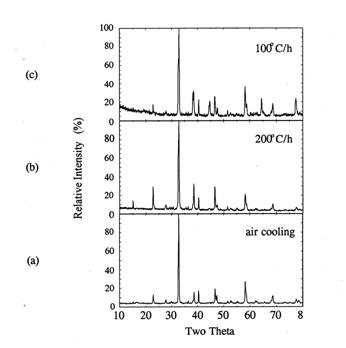
Figure 6. XRD of the
silver-sheathed samples which were heated to 950℃,
held for 30 minutes and
cooled at different cooling rates.
Figure 7 compared the critical transition temperatures(Tc)
for samples cooled at air quenching and 100℃/h. The air quenched sample shows that
an onset temperate for superconductor(Tc onset) was about 70K and
ΔTc was about 15K. From TGA result in figure 5, the oxygen
content of 1 ㎜
thick sample after 30 minutes holding at 950℃ was approximately 6.5. Orthorhombic Y123
phase having Y: Ba: Cu: O = 1: 2: 3: 6.5 shows the Tc onset≈
60-70K [14]. Therefore, the Tc onset in this case is consistent with
the value from TGA result. When the sample was cooled 100℃/h, Tc onset was
about 92K and ΔTc within 5K. This result suggests that the
total oxygen content of the sample is close to 7. The oxygen content of 7 is
consistent with XRD results.
XRD, TGA, and Tc results indicates that when a
bulk sample of 1㎜
thick was soaked for 30 minutes at 950℃ and cooled at 100℃/h in air environment,
additional oxygenation process is not necessary to form 92K Y123
superconductor. This reaction time for the formation of 92K Y123 superconductor
is much shorter time than that of other case. Okada et al.[15] reported that
Y123 superconductor in silver-sheathed samples was obtained through both
calcination at 950℃
for 5 hours and thermomechanical processing at 910℃ for 5 to 150 hours in
oxygen environment.
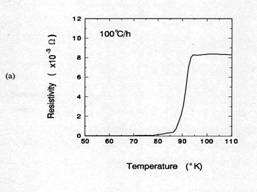
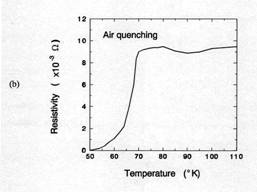
Figure 7. TC of
silver-sheathed samples. Sample having 1mm in thickness were
(a) air quenched and (b)
cooled at 100℃/h
in air after soaked 30 min at 950℃.
3.3
Melt processing of pellet samples
Melt processing has been used to improve the transport Jc
by obtaining a very dense and preferentially aligned microstructure. In
this study, it was possible to improve the superconducting quality during melt
processing because orthorhombic Y123 could be fabricated from high oxygen
precursor without additional oxygenation process. We conducted two series of
experiments. In these series of experiments, the precursor was formed into 0.27
㎝
thick and 1.25 ㎝
diameter pellets. In the first series, holding time at 1020℃ was varied from 2~30
minutes to find the minimum reaction time for the formation of the orthorhombic
Y123. In the second series, cooling rate was varied from air quenching to 5℃/h.
3.3.1
Reaction time for formation of orthorhombic Y123 at 1020℃
The samples were rapidly heated to 1020℃, held for 1,5, 10, 20, or
30 minutes in air, and quenched in air. Figure 8 shows the XRD patterns of the
melt processed samples for each processing conditions.
After 1 minute holding, some amount of orthorhombic Y123
phase was formed. The Y2O3, BaCuO2 and CuO
were remained as the major phase. After five minutes of reaction at 1020℃, orthorhombic Y123 was the
predominant phase and BaCuO2 and CuO phase were still remained.
Small amount of 211 phase was also detected in XRD. As the reaction time
increased more than 5 minutes, orthorhombic Y123 peaks increased and the
impurity peaks decreased appreciably. After 10minutes of reaction, the
orthorhombic Y123 phase was formed more than 95% and only trace of 211 and
BaCuO2 were remained. When reaction time increased to 30 minutes,
almost single orthorhombic Y123 phase was formed with trace of 211 phase.
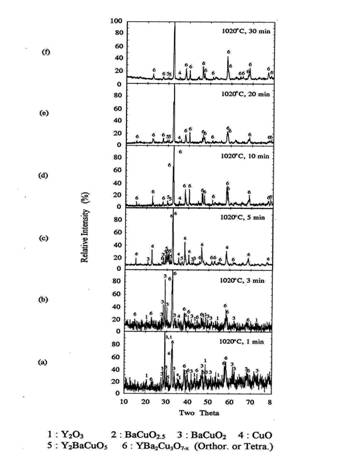
Figure 8. XRD of the melt
processed pellets at 1020℃
for 1-30 min
and
quenched in air.
We think that trace of 211 and BaCuO2 phases
after 10 or 30 minutes holding are related to the melting of the Y123 phase
because of the following observations. First, the sample showed melting after
5~30 minutes heat treatment at 1020℃. Second, as shown in XRD patterns,
orthorhombic Y123 phase started to form even after 2 minutes of reaction and became
the primary peak after 5~30 minutes holding. It is well known that Y123 melted
incongruently to 211 plus a Ba-Cu rich liquid[16]. During cooling process, a
fast cooling cause a incomplete reverse reaction. The incomplete reaction leads
to the presence of 211 and the liquid phase of BaCuO2 and CuO.
Therefore, the above results indicate that the minimum reaction time for the
formation of orthorhombic Y123 at 1020℃ was about 10-30 minutes.
3.3.2 Cooling Rate Effects at 1020℃
In this series experiments, the samples were held for 30
minutes at 1020℃
and subsequently cooled at two different rate. Samples were cooled at 100℃/h to room temperature or
at 5℃/h to
920℃
and at 100℃/h
to room temperature. Figure 9 shows the XRD patterns of the samples. For the
comparison purpose, the XRD pattern for the air quenched sample was added in
(c).
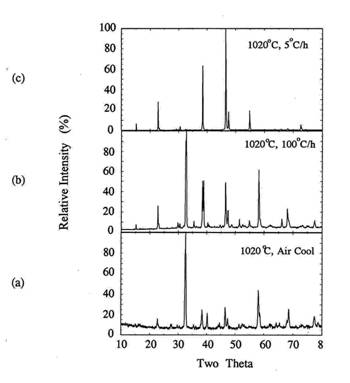
Figure
9. XRD for the melt processed samples at 1020℃ with different heating rates.
(a) 100℃/h,
(b) 5℃/h
to 920℃
and 100℃/h,
(c) air quenching.
In all cases, the formed Y123 phases were orthorhombic.
As the cooling rate decreased from melting temperate, c-axis texturing in (001)
peaks became predominant. The better grain alignment with slower cooling rate
is related to the mass transport rate at the solid-liquid interface during melt
processing. Since the a-b plane is considered to be fast growth plane in Y123
superconductor[14], slow cooling rate around the melting temperature allows
sufficient time for mass transport along the ab plane and for nucleation along
the c-axis to occur.
4. CONCLUSIONS
1.
The synthesized intermediate precursor(IP) powder containing BaCuO2.5,
Y2O3 and CuO was successfully converted to orthorhombic
Y123 superconducting phase within 10 minutes at 954℃ when the powder was
rapidly heated to the peak temperature.
2.
For 1mm thick silver-clad samples, if the sample was soaked for 30 minutes at
950℃ and cooled at 100℃/h
in air, additional oxygenation process was not necessary to form 92K Y123
superconductor. From TGA result, this fast formation 92K superconductor is
presumably due to the direct conversion of BaCuO2.5 to orthorhombic
Y123 at high temperature.
3.
In the melt processing at 1020℃, the reaction for the
formation of orthorhombic Y123 in the 2.7mm thick IP pellet sample was
completed within 30 minutes. At the cooling rate of 5℃/min
to 920 ℃ and 100 ℃ to
room temperature in air, orthorhombic Y123 superconducting phase was obtained
without an additional oxygenation process at low temperature.
4.
As the cooling rate decreased from 100℃/min
to 5 ℃/min between the melting temperature
and 920℃, c-axis texturing in (001) peaks
became predominant due to faster mass transport rate along a-b plane and thus
faster grain growth.
REFERENCES
1.
G.
Selvaduray and C. Zhang, J. Mater. Res.
7, 283 (1992)
2.
T.
Nevriva, P.Holba, S.Durcok, D.Zemanova, E.Pollert and A.Trisk, Physica C, 157, 334 (1989)
3.
T.Aselag
and K. Keefer, J. Mater. Res., 3, 1279 (1988)
4.
M.Murakami,
Superconduc. Sci. Tech., 5, 185 (1992)
5.
J.L.
Taloni, D.M. Pooke, M.P. Staines, M.E. Bowden, N.E. Flower, R.G. Buckley, M.R.
Presland and R.C. Davis, Physica C, 171, 61, 1991
6.
7.
H.G.
Lee and
8.
D.E.
Morris, A.G. Markelz and J.H. Nickel, Physical
C, 168, 153 (1990)
9.
K.
Hishio, K. Suzuki, T. Hasegawa, T. Yamamoto, K. Kitazawa and K. Fueki, Solid State Chem., 82, 192 (1989)
10.
T.
Aselage and K. Keefer, J. Mater. Res.,
3, 1279 (1988)
11.
T.
Umemura, K. Egawa, M. Wakata and K. Yoshizaki, Jpn. J. Appl. Phys., 28,
L1945 (1989)
12.
K.N.
Tu, N.C. Yeh, S.I. Park and C.C. Tsuei., Phys.
Rev. B, 38, 5118 (1988)
13.
14.
K.
Salama, V. Selvamanickam and D.F. Lee, In processing and properties of High-Tc
Superconductors, eds. S. Jin, 155, Singapore, World Scientific (1993)
15.
M.
Okada, A. Okayama, T. Morimoto, T. Matsumoto, K. Aihara and S. Matsuda, Jpn. J. Appl. Phys., 27, L185 (1988)
[ BWW Society Home Page ]
© 2006 The BWW Society/The Institute for the Advancement of Positive Global Solutions