The Sciences: Chemistry:
Heptachloro-1.4-Dioxane MP 57°C:
A Compound of Merely Academic Interest
by
Central Institute of Chemistry, Universidade Federal de Santa Maria (UFSM), Santa Maria, Rio Grande do Sul, Brazil
Editor’s Note: In two essays (1, 2), the author wrote about halogen-substituted 1.4-dioxanes, namely "conversion isomerism" from a stereochemical point of view and the academically interesting question of the “dependence of the insecticidal effect on the spatial construction of the synthesised dioxane derivatives.”
The question repeatedly asked in this connection is whether or not the heptachloro-dioxane discussed could be classified as an insecticide was briefly addressed in passing and will be answered below.
Between 1943 and 1947 the author synthesized more than 400 halogenated compounds for systematic research on “constitution (configuration) and insecticidal activity” with the aim to detect new active representatives in this class (3, 4). Among these compounds was also a series of previously unknown halogenated 1,4-dioxanes (5a,b): the heptachloro-dioxane (I-), mentioned in the title together with its octachloro derivative [(X) in Table 1] and a hexachlorodioxen (II) resulting from I- by HCl-splitting [1944 (5a), 1947 (5b), Plate 1 (5c)].
In parallel running research the author was interested in several stereochemical problems on “uneven six-ring compounds” especially on dioxane- and cyclohexane-substitutionproducts. So the above mentioned I- came into game once more: since 1945 the author had been looking for the second I-isomer - theoretically possible in case of chair dioxane form: Figure 1 (16):
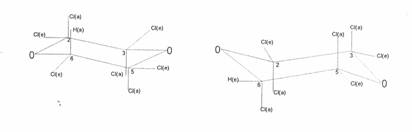
The two theoretically possible chair configurations of heptachloro-1,4-dioxane:
6a(H)2ea3ea5ea6e(Cl) and 6e(H)2ea3ea5ea6a(Cl)
In the beginning of the 1950’s W. Stumpf, of the University of Heidelberg, was believed to have isolated two I-isomers as he described in his habilitation paper and in (6a,b), but he was mistaken as we could confirm within 40 years research about dioxane-chlorination products: Only one I-isomer, namely the one of mp 57°C, can be isolated by chlorination of dioxane and of its chlorination products.
A further error in the
In co-operation with the following nominated colleagues and Institutions in Germany, Japan and Brazil we found that I- mp 57°C and I-crude products (“hepta oils”) prepared in FU Berlin were not useful for chemical pest control as far as tested between 1951 and 1958, with I-material, including “hepta-oils” delivered from the author:
- Plant Protection Department of HOECHST,
- Prof S Takei, Agricultural Chemistry
Institute,
- Prof R Wasicky, Pharmaceutical Institute,
- Malaria research institute in
-
Dr K Schulze, director
of the Biological Department of RIEDEL
I- and I-formulations: tested in the Malaria Institute in
Summarizing all test results from (7) to (11) one can say:
Heptachloro-1,4-dioxane mp 57°C (I) cannot be classified as insecticide comparable with DDT, Gammexane, M 410.
I- and its analogues are only of academic interest, for instance for systematic research “dependency of insecticidal activity of spatial structure” : Table 1 (5b).
Via the I-dehydrohalogenation product II the heptachloro-1,4-dioxane mp 57°C (I) offered the entrance into the chemistry of F- and Cl-substituted dioxanes and dioxenes: Plate 1 (5c).
Among the authors misled by the publication of Stumpf were K. Hayduk and K. Lüdicke of Heidelberg University, whereby they took the view that heptachloro was an insecticide comparable to DDT and gammexane. In an essay (12) titled "The Significance of Physical Factors for the Effect on Heptachloro on Insects" the two authors underlined this view. However, one would have expected from a publication geared to physics that the heptachloro compound used would at least have been characterized by a melting point. Nothing of the kind - only the information: "Compound provided by the plants HÜLS or BASF, respectively". A purity test of the I- compound used would have been necessary for the sole reason that a second heptachlorodioxane isomer was mentioned at the time. Then, the authors even had to expect a second isomer because it took us many years of experimentation to disprove its existence (1,2)
The comparison with gammexane creates the impression that heptachlorodioxane is an insecticide of significance. The result of their experimental tests was "The pictures of poisoning caused by DDT and heptachlorodioxane show a certain conformity."
The working instructions for heptachlor-1,4-dioxane (I) and hexachloro-1,4-dioxene (II), including Plate 1 which gives an impression of the prepared follow products:
Heptachloro-1.4-dioxane (I), C4HO2Cl7 :
Chlorine gas is introduced with stirring into a mixture of 2.5 kg of high purity dioxane and 1 kg of CCl4. The temperature increased first to 65°C, then to 80°C (reflux condenser). After the usual work-up, an oily distillation residue is obtained from which I- separates in a crystalline form melting at 46°C. Pure recrystallized I- melts at 56°-57°C [(656, 657) in (15)]
On the basis of this method, Farbwerke HOECHST produced major preparations of I- on our behalf, permitting us to carry out the following experiments:
Dehydrohalogenation
of I to hexachloro-1,4-dioxene (II), C4O2Cl6 :
![]()
2500 ml of methanolic NaOH containing 200 g of NaOH are gradually added with stirring to 300g of I dissolved in 500 ml of CCl4 at 5°-10°C. The splitting-off of HCl is stopped by dilution with H2O to a volume of 10 litres. Work-up resulted in 199 g of II having a boiling point of 67° - 70°C (2 Torr), pure II melts at 20°-22C,
n : 1,5211 [(657) in (1)].
Plate 1:
Formulas of the Cl- and F-substituted 1,4-dioxanes, -dioxenes and -dioxadienes (5c)
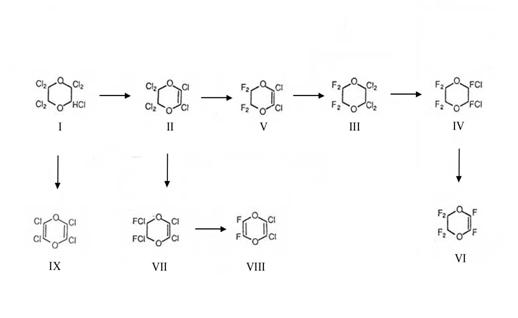
Experimental data for the preparation of compounds III – IX gained during the years 1944-49 in PROJ X 3,2 (15); see also Table 1 (5b)
Summary:
I mp 57°C had never any practical meaning for Pest Control (7-11) as you may think reading older publications e.g. (6,12).
I- mp 122°C does not exist (1,2).
Bibliography:
(1) R Riemschneider
About the so-called heptachloro-1,4-dioxane from mp 122°C
http://www.bwwsociety.org/journal/html/bcompounds.htm 2005;
cf. APPENDIX X 4,2 in (15)
(2) R Riemschneider
The significance of proving the non-existence of heptachloro-1,4-dioxane from mp 122°C, published 2006 at http://www.bwwsociety.org/journal/html/heptachloro.htm
cf. APPENDIX X 4,3 in (15)
(3) R.Riemschneider
Zur Kenntnis der Kontakt-Insektizide I“,
2. Beiheft zur Zeitschrift „Die Pharmazie“ 1947 Verlag Dr.Werner Saenger, Berlin(OST)
(4) PROJ VI-XIII in (15)
(5a) R. Riemschneider, K. Schürken (1944)
About preparing superchlorinated 1,4-dioxanes: C4HO2Cl7, C4O2Cl8, "hepta oils"
Lab report, RUHRÖL GmbH Bottrop, May 44, 6 p
(5b) R. Riemschneider, K. Lohmann, W. Gerischer, W. Cohnen,
A. Heymons (1947 – 1949)
About preparing and identifying 10 Cl-substituted 1,4-dioxanes, -dioxenes [and subsequent products (Tab 1)] as well as testing for contact-insecticidal activity (direct chlorinations of 1,4-dioxanes and 1,4-dioxane chlorinated products); heptachloro-1,4-dioxane from mp 56-57°C, octachloro-1,4-dioxane from mp 110-112°C.
Bull Physiolog Chem Inst
Table 1: Contact-insecticidal activity of saturated halogenated dioxanes and unsaturated halogenated dioxenes1 on Calandra granaria in filter test avoiding respiratory poison effect (contact poison test apparatus II)2 [Nov 1953]
Test substance relative effectiveness 3
tetrafluorotrichlorodioxanes C4HO2F4Cl3 ++
heptachlorodioxane (I) C4HO2Cl7 ++
hexafluorodichlorodioxanes C4O2F6Cl2 +
monofluoroheptachlorodioxane C4O2FCl7 (+)
octachlorodioxane (X) C4O2Cl8 (+)
For comparison:
p,p`-DDT Cl14H9Cl5 ++++
p,p`-DFDT Cl14H9F2Cl3 +++++
____________________________________ __________________________________________
1 The halogenated dioxenes, not listed in this table can be found in Table 2 in (2); they are inactive under these conditions3
2 Contact poison test apparatus II: Avoids respiratory poison effect by directing the air stream through closed, parallel sintered-glass nutsch filters.
Insects and test substances: in each filter there were 30 Calandra granaria on disks containing the test substances (applied in an acetone solution of defined concentration, dried on needle stands).
3 Relative effectiveness when applying 500 μg / cm2
(5c) R Riemschneider, F Scherer, W Stuck
Preparing flourinated chloro-1,4-dioxanes, -dioxenes, -dioxadienes and testing for contact-insecticidal activity: Plate 1
Ms 1953, 17 p: Tab 1 and 2 in (2)
Free Univ of
Further experimental details to Plate 1 in (15). There Special Part at X 3.2.
The mentioned manuscript was sent confidentially to Prof Dr S Takei, Kyoto Univ. Copy of letter in (15).
(6a)
Chemistry and applications of 1,4-dioxane
Verlag Chemie GmbH, 1956, p 130-133
This publication is based on Stumpf’s habilitation paper, directed by K. Freudenberg, University Heidelberg
(6b) F Scherer, R Riemschneider (discussant), R Wasicky
Critical comments during discussion of
lecture by
Chemists
Congress,
Text of
comments sent to Österreichische Chemiker-Zeitung,
In the discussion after this lecture the author doubted already then the existence of the mentioned heptachloro-1,4-dioxane mp 122°C;
It seemed very improbable, after our extensive experience in the field of Hal-substituted dioxanes (and cyclohexanes), that a symmetrically build octachloro-dioxane mp 110-112°C (X) would melt at a lower temperature than the so-called heptachlorodioxane mp 122°C. We prepared already in 1944 and 1948 heptachloro-1,4-dioxane mp 57°C and X (5a,b).
(7) Farbwerke HOECHST , Plant Protection Department
I pure delivered by FU Berlin, Dozent Dr.R.Riemschneider
Results: no further experiments intended
(8) S.Takei, M.Nakajima,
Institute for Agricultural
Chemistry .
1953-1955 (unpublished)
Results: I without practical meaning as insecticide.
(9) R.Wasicky, O.Unti
Pharmaceutical Institute, Universidade S.Paulo, Brasil
Heptachlorodioxane (I) experiments - including insecticidal test experiments [method as described in (14)]
1953-58, unpublished
I cannot be classified as an insecticide
(10) R.Wasicky - in co-operation with the Malariaforschungsinstitut,
Rio de Janeiro
Versuche der Bekämpfung der Anopheles Mücke mit Heptachlordioxan (I) Ergebnisse: negativ
I geliefert vom Autor, FU.
(11) K.Schulze, Leiter der Biologischen Abteilung der Firma RIEDEL de HAEN, Berlin-Britz
Testversuche mit Heptachlordioxan (I) an Seidenraupen, Bombyx mori, und anderen Insekten
Ergebnis: I zeigt nur mässige insektizide Wirkung
I geliefert vom Autor, FU.
(12) K.Hayduk , K.Lüdicke
Die Bedeutung physikalischer Faktoren für die Wirkung von
HEPTACHLORDIOXAN auf Insekten
Z.Naturfoschg.11b , 383-388 (1956)
Kommentar auf Grund von Nacharbeitungsversuchen, durchgeführt 1957 von W.Gerischer im Auftrag des Autors, FU:
In a reproduction by our staff with experience in handling insecticides, especially Ms. W. Gerischer, the test method used for determining the contact-insecticidal effect of the investigated compound in "wooden boxes with films making time-consuming cleaning superfluous" turned out to be totally useless as far as the issue of "contamination" was concerned. Removing the last gammexane residues from glass vessels requires a treatment we tested over many years and described to many interested users upon request. At the time, we did not always succeed in cleaning wooden containers completely. Gammexane used for comparative purposes would have required working in glass vessels at all times (13).
In this case, the investigators compared a compound with a weak effect with the highly active gammexane, forgetting that gammexane was also called "the atom bomb in the insect world".
(13) W Gerischer
Wie reinigt man Geräte? Pharmazie 4, 481 (1949)

(14) R.Wasicky,O.Unti
Dichlorodifeniltrichloroetano no combate as larves de culicideos
Arch.Hig.Saude Publica 9, 89-102 (1944) ; ref: Rev.appl.Entomol 33B, 77 (1945)
(15) R.Riemschneider
“Re-reading – 66 years chemistry” PROJ I – XXVI (in preparation)
(16) R Riemschneider
About configurations of 1,4-dioxane substitution products
Z Naturforschg 8b 745-751 (1954), Free Univ of
English translation in PROJ X 4,1a in (15).
Correspondence to: rriemschneider @yahoo.de
[ BWW Society Home Page ]
© 2008 The Bibliotheque: World Wide Society