Medicine: Psychiatry:
The Eternal Now:
Towards an Interdisciplinary Theory of Schizophrenia
By Professor Bernhard J. Mitterauer, Institute of Forensic Neuropsychiatry and Gotthard Günther Archives,
University of Salzburg, Austria
Abstract: This paper presents a
new explanatory model for schizophrenia based upon molecular and
neurobiological hypotheses, Barbour´s theory of the universe and years of
experience in observing and treating these patients. I start out with the
psychiatric hypothesis that in delusions and hallucinations the self-boundaries
are lost. First, the concept of the Self is defined as a self-reflective system
consisting of many subsystems in the brain striving for self-realization in the
environment. Whenever a system treats non-realizable programs as if they were
realizable its ability to "test the reality" is lost, and
consequently a loss of self-boundaries may occur. On the molecular level I will
try to show how "non-splicing"
of introns during the mRNA splicing process is equivalent to a loss of the
rejection of non-realizable programs. At the cellular level in the brain, I
have in previous work attributed a spatio-temporal boundary setting function to
the glial cells such that glial cells determine the grouping of neurons into functional
units. Mutations in genes that result in non-splicing of introns can produce
aberrant versions of neurotransmitter receptors that lack protein domains
encoded by entire exons and can also have protein sequence encoded by introns
that have not been properly spliced out.
I propose that such "chimeric" receptors are generated in
glial cells and that they cannot interact properly with their cognate
neurotransmitters. The glia will then lose their inhibitory-rejecting function
with respect to the information processing within neuronal networks. The loss
of glial boundary-setting may result in a 'borderless' generalization of
information processing such that the structuring of the brain in functional
domains is almost completely lost. This loss of glial boundary setting could be
an explanation of the loss of self-boundaries in schizophrenia. The loss of
temporal boundaries may cause a sense of an “eternal Now” in the brain of
schizophrenics, comparable to the theory of Barbour. Pertinent examples of case
studies are given attempting to deduce the main symptoms of schizophrenia from
the proposed hypotheses. Tying all arguments and hypotheses together, an
interdisciplinary definition of the etiology of schizophrenia is attempted.
Key words: interdisciplinary theory
of schizophrenia – non-splicing – non-rejection - loss of glial
boundary-setting function – loss of self-boundaries – timeless self
Introduction
The
primary symptoms of acute schizophrenia for which an explanation must still be
sought include the occurrence of hallucinations, delusions and thought disorder
(Frith, 1979;1987; Hemsley, 1982; 1987; Gray, 1991). There are numerous
hypotheses concerning the etiology of schizophrenia, most of which focus on
biological, psychological and sociological factors (Carpenter and Buchanan,
1995; Johnstone et al, 1999; Maynard et al, 2001; Shastry, 2002). However, the
presumption, or even conviction, that schizophrenia has a multifactorial
etiology arising from a combination of various factors seems to force itself
upon most clinically versatile psychiatrists. Explanatory models of this
psychobiological disorder should, therefore, be based upon an interdisciplinary
approach. My explanatory approach to the schizophrenic disorder and
symptomatology is based upon molecular and neurobiological hypotheses as well
as on years of experience in observing and treating these patients. From a
physic point of view I draw parallels between the schizophrenic brain and
Barbour´s theory of the universe (Barbour, 1999).
Psychiatrists generally explain delusions and
hallucinations in terms of a “loss of ego boundaries” or “inner/outer
confusion” (Fisher and Cleveland, 1968; Sims, 1991)) Instead of the rather
uninformative concept of ‘ego’, I introduce a neurophilosophical concept of the
Self. According to the theory of subjectivity of Günther (1962), the
boundary-setting between the Self and Non-Selves is essentially based on a
rejection function or mechanism. Supposing that schizophrenic symptoms are due
to serious brain dysfunction with a biological basis (Werner et al, 1982;
Heyman and Murray, 1992), it should be possible to find out how this
dysfunction on a molecular and cellular level may occur.
On the molecular level it will be shown in the
context of mRNA splicing that a “non-splicing” of introns is equivalent to a
loss of the rejection function (Mitterauer, 2001). This in turn leads to the
production of an aberrant protein, which in some cases could result in a
chimeric receptor being expressed on the surface of a neuron or glial cell (see
section 3).
On
the cellular level, I will focus on glial-neuronal interaction by attributing
to glial cells a spatio-temporal boundary-setting function such that the glial
cells determine the grouping of neurons into functional units (Mitterauer,
1998; 2000a; Mitterauer et al, 2000). If we suppose that “chimeric” receptors
are generated and expressed on the surface of glial cells, these receptors
cannot be occupied by their cognate ligands resulting in glia that lose their
inhibitory-rejecting function with respect to the information processing within
neuronal networks. This boundary loss, which may occur in localized regions in
normal individuals, affects many brain areas simultaneously in schizophrenia
(for review see Lewis, 2000), such that the brain structuring in functional
domains is almost completely lost. This neurobiological explanation for a
borderless generalization of neuronal information processing can be applied as
an explanatory model for the “loss of self-boundaries” in schizophrenia. Since
the loss of self-boundaries concerns both space and time, the schizophrenic
brain embodies not only an infinite universe, but also a timeless universe.
This state of existence is comparable to Barbour´s universe, in which time has
no reality and everything is now.
After
elaborating these hypotheses in detail, I will discuss the clinical
correlations and finally attempt an interdisciplinary definition of the
etiology of schizophrenia.
2.
The concept of the Self
2.1.
Self-reflection
In discussing a “loss of self-boundaries” it is necessary to at least
attempt to define the concept of the Self. Generally speaking, the prefix
‘Self’ points out that a system is capable of formal recursion or mechanic
feedback. On higher levels, as in the human brain, terms like self-observation
or self-consciousness are based on the ability of reflective thinking. In
reflective thinking a thought becomes the object of the same computation-like
process. A distinction from recursion lies in the fact that in the process of
reflection additional new arguments may enter the process. Reflective thinking
is the activity of a participant observer constituted as a Self acting as
initiator, participant and observer of the process. Self-reflection is a
generalization of this situation insofar as a Self, which is the subject of
first-person thoughts and first-person reference, posits itself as a thought
(object) for reflective thinking. The agent in self-reflection is not only
subject and object (argument and outcome) of the reflective process, but – as
part of reflective thinking – it is also able to say “I am thinking this and
that; which is my Self”. Therefore, a theory of the Self implies the problem of
consciousness.
2.2. Neurophilosophical approaches
I have postulated that self-reflection may be represented in a manifold
manner in the brain (Mitterauer, 1998), similar to the self-systems of “Many
Cartesian Theaters” (Damasio, 1992), which normally do not reach
self-consciousness. Presumably, these are places in the brain where information
is stored in relation to specific areas of reality (persons, experiences,
etc.). Most of the spatio-temporally limited structures of the brain are
probably used to process current information, but the allocation of
neurobiological operational figures can steadily change. Although we know that
certain areas of the brain have priority for special tasks, it has not been
possible to find a topological allocation for the different and complex
reflection processes or to localize self-systems.
We should, however, be able to find the part of the brain that
integrates all of the different self-systems in the undisturbed
self-consciousness. Several decades ago, the group working with McCulloch
considered the formatio reticularis in the brain stem as an ‘integrative
matrix’ (Scheibl and Scheibl, 1968) as well as a ‘central command system’
(Kilmer et al, 1969). Lately, Newman (1997) has suggested an ‘extended
reticular-thalamic activating system’ as a neural correlate for a ‘central
conscious system’ (see also Steriade, 1996). According to Churchland (2002),
the most basic level of inner coordination and regulation occurs in the brain
stem , anchoring what Damasio (1999) refers to as “the protoself”.
From an anatomic-functional point of view, Baars (1996) provides
numerous examples (e.g. split-brain patients, sensorimotor homunculus) which
show that different self-systems can be represented in the brain: “The nervous
system abounds in such maps, some of which appear to serve as ‘self-systems’,
organizing and integrating vast amounts of local bits of information”. Damasio
(1994) refers to a ‘neuronal self’. In this view, a neuronal system capable of
producing subjectivity must be equipped with sensory and motor cortical areas
as well as subcortical nuclear areas (thalamus, basal ganglia) having
convergence properties. Edelman (1992) is also of the opinion that there is a
biological self which he confines to subcortical homeostatic systems. It is
questionable whether such a local allocation is possible for ‘self-systems’.
(Concerning the synchronization hypothesis of consciousness and the
chaos-theoretical approach see Mitterauer, 2000a).
2.3. Self-realization
A Self is always the Self of
a living system having biological “hungers” (Iberall and McCulloch, 1969) or
intentions. Therefore, a living system permanently strives to realize its
intentional programs in an appropriate environment. According to the logic of
acceptance and rejection (Günther, 1962), appropriate objects may be accepted,
but inappropriate objects must be rejected.
Table
1. Interpretation of Basic Schizophrenic Symptoms
|
Loss of Boundaries |
Symptoms |
|
–
conceptual |
–
thought disorder |
|
–
ontological |
–
delusions |
|
–
perceptive |
–
hallucinations |
|
–
motoric |
–
catatonic symptoms |
|
–
emotional |
–
affective flattening |
Table 1 shows an example of an intentional program (1, 3, 2, 4),
which either accepts or rejects objects (1, 2) in the environment. Let
us suppose that a subject is trying to realize its intentional program by
moving through an environment in four steps. The intentional values (iv) are 1,
3, 2, 4. Two objects, with their respective object values (ov) 1, 1,
are found in the environment. In the first step, the detected object (ov) 1
can be accepted. In the second step, the intended value (iv) 3 rejects
both objects (ov) 1, 2. In the third step, one of the two objects (2)
corresponds to the subject´s intention, hence it accepts the object. In the
fourth step, finally, the intended value (iv) 4 results in another
rejection of both objects (ov) 2, 2.
Without doubt, to stay alive
we must often change our intentional program adjusting it to the environment.
However, if we would do so constantly, such a slavish behavior would occur at
the cost of our individuality. The intentional programs of self-conscious
systems such as the human one are highly specified by their genomes and
experiences during life history. Therefore, to cope with individuality, a Self
must be able to reject non-realizable programs at a point of time, in the sense
of self-realization. One could also say that the rejection mechanism is
essential for setting boundaries between the Self and the many Non-Selves.
Understood in this sense, the question arises as to
what makes a Self or a brain incapable to reject the inappropriate and to set a
boundary between itself and the environment.
3. The loss of self-boundaries
3.1. Molecular hypothesis
3.1.1. The splicing mechanism
For
a better understanding of my interpretation of rejection, I would like to refer
to the example of the structure and protein producing function of a gene. Most
genes of higher living beings are constructed such that protein coding
sequences (exons) alternate with non-protein coding sequences (introns).
However, the chemical structure of nucleotides that make up exons and introns
is the same, rendering them isomorphic even though with respect to the
realizability of their genetic coding they are different. The genetic code of
the exons is realizable, since it leads to a production of proteins. The genetic
code within the intronic sequences is not realizable since it does not supply
an instruction for the production of a protein. Introns are therefore spliced
out of mRNA shortly after mRNA synthesis. Theoretically speaking, this means
the rejection of non-realizable programs.
3.1.2.
Non-splicing of
introns, alternative and abnormal splicing
As
already mentioned, virtually all genes of higher organisms have non-coding
introns interspersed between the coding exons that must be spliced out in order
to generate a mature messenger RNA molecule used to encode proteins. I have
taken the novel approach of considering the process of splicing to be a
rejection mechanism. Mutations in genes that control the splicing process of
other genes can have serious consequences to the organism because the correct
splicing patterns are not obtained. In some cases exons are spliced out when
they should be retained, and in other cases introns are retained when they
should be spliced out. Non-splicing is particularly serious and is essentially
the loss of the elementary rejection mechanism in which introns that must be
rejected, or spliced out, are not. The resulting messenger RNA thus contains
introns and when this mRNA is used to encode protein, the inappropriate
intronic sequence results in the production of a faulty protein product. The
protein will have additional amino acid sequence that will usually disrupt its
proper functioning. Furthermore, an unspliced intron will often have an inframe
stop codon that will generate a premature truncation. The truncated proteins
lack their carboxy-termini and are often unstable and shortlived, so these
proteins are usually non-functional as well.
A
variation of the splicing mechanism is alternative splicing. Alternative RNA
splicing means that a cell can splice the primary transcript in different ways
and thereby make different polypeptide chains from the same gene. Alternative
splicing can produce different proteins with normal or abnormal functions
(Clark et al, 2002). Abnormal splicing is based on a “splicing error”, which
was already found in brain tissues of schizophrenic patients (Liu et al, 1994;
Schmauss, 1996). This research group identified truncated dopamine receptors in
the brains of chronic schizophrenic patients. Abnormal splicing could play a
role in subgroups of schizophrenics, but the decisive argument of my hypothesis
is that a non-splicing of introns is at least responsible for the positive or
productive symptoms, such as delusions and hallucinations.
3.2.
Neurobiological
hypothesis
3.2.1.
The boundary-setting
function of the glial system in its interaction with the neuronal system
Glia
have been shown to play an important role in the inactivation of
neurotransmitters (Martin, 1995; Sykova et al, 1998). Glia divide the brain
into compartments on the one hand, and create functional units in various time
scales with the neurons, on the other hand (Mitterauer et al, 1996). I have
hypothesized that the glial networks have a boundary-setting function in their
interaction with the neuronal networks (Mitterauer, 1998). Various experimental
indications support the hypothesis that the glial-neuronal networks are
organized in the form of compartments in which the glial cells are responsible
for the formation of self-organizing units (Mitterauer, 1998; 2000a). Glial
cells (particularly astrocytes with their processes that contact or even enfold
a synapse) modulate the “efficacy of synaptic transmission” (Teichberg, 1991;
Mitterauer et al, 1996; Sykova et al, 1998; Mitterauer, 2000a; Oliet et al, 2001;
Fields and Stevens-Graham, 2002). Signals between astrocytes and neurons can be
mediated by glutamate (Gallo and Ghiani, 2001), acetylcholine (Smit et al,
2001) and other neurotransmitters (Kimelberg et al, 1998). GABA is released
from glial cells upon glutamate receptor stimulation (Gallo et al, 1989) and
inhibits neuronal signalling. Also involved are complex ionotrophic and
metabotrophic interactions (Teichberg, 1991; Reichenbach et al, 1998).
Intracellular calcium oscillations in astrocytes have been hypothesized to
affect synaptic cleft calcium concentrations (Cooper, 1995; Newman and Zahs,
1997) and, subsequently, the amount of neurotransmitter released from
presynaptic terminals. In addition to modulating synaptic transmission in
neuronal cells, astrocytes may play a direct role in generating pacemaker
rhythms (Mitterauer et al, 2000).
This
originally speculative assumption was recently experimentally verified. Parri
et al (2001) showed that astrocytes in situ can act as a primary source for
generating neuronal activity in the mammalian central nervous system. Slow
glial calcium oscillations (every 5-6 minutes) occur spontaneously and can
cause excitations in nearby neurons. Although there is experimental evidence
that neuronal-glial interactions also occur in the millisecond range (Murphy et
al, 1993; Mennerick et al, 1996), until now rapid glial oscillations within a
second have not been found. Following the rationale of my brain model, rapid
glial oscillations might be observed in the near future. It is notable in this
respect that short (about 1 min.) song rhythms of Drosophila are controlled by
the clock gene per, whose expression is observed only in glia (Hall,
1995).
The
neuronal principle of compartmentalization (Rall, 1995) may also be applied to
glial-neuronal interaction if one assumes that glia divide or combine neurons
in functional units (Varon and Somjen, 1979). Steindler (1993) describes
transient glial boundaries that surround functional groups of neurons, their
dendrites, and axons during neural development, referred to as “cordons”.
Another convincing experimental result indicates that glia have a
boundary-setting function and that this function divides the neuronal system
into spatio-temporal compartments (Mitterauer, 1998). Recently, it has even
been demonstrated that the number of synapses is controlled by glia (Ullian et
al, 2001) and that glial protein S100B modulates long-term neuronal synaptic
plasticity (Nishiyama et al, 2002). But also a glial cell itself may consist of
hundreds of independent compartments (“micro-domains”) capable of autonomous
interactions with the particular group of synapses that they ensheath (Grosche
et al, 1999). According to Smolin (1997), the setting of boundaries is an
absolute prerequisite for a description of the universe. The universe can only
be described if we set boundaries around regions and describe what is inside in
terms of information that is associated with each boundary.
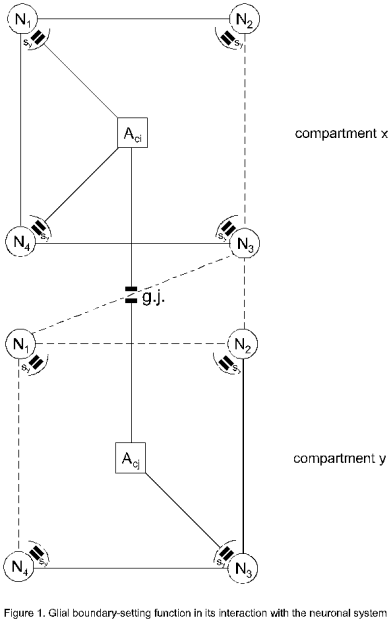
Two astrocytes (Aci, Acj) which can activate or
deactivate n-neurons (N1…N4) via their processes are
displayed in Figure 1. Here the neurons are interconnected in the sense of a
neuronal network. The interactional structure of an astrocyte with n-neurons
can be defined as an elementary compartment of nerve cells (compartment x,y).
By simultaneously regulating neurotransmission in all of the synapses (sy)
enveloped by an astrocyte, the astrocyte calcium wave may coordinate synapses
into synchronously firing groups (Antanitus, 1998). Depending upon the number
of neurons connected with the processes of astrocytes, various combinations of
neuronal activation or deactivation are possible generating a big potency of
information structuring. I call this glial function ‘spatio-temporal boundary setting’ in neuronal networks. Figure 1
shows two simple examples. In compartment x the astrocyte is activating only
two neurons (N2, N3, thick lines). In compartment y three
neurons are activated (N1, N2, N4, thick
lines). This implies that in the neuronal network only these activated neurons
are ready for information processing. Compartments are linked via gap junctions
(g.j.). Gap junctions are essential to astrocytic signalling function and it is
estimated that more than 50,000 gap junction channels interconnect each
astrocyte with its neighbours (Cotrina et al, 2001). In addition to neuronal
networks these gap junctions form glial networks (syncytium). A glial syncytium
can structure information provided by a local Ca 2+ wave into a
distinct spatial and temporal pattern of Ca2+ oscillations
(Strahonja-Packard and Sanderson, 1999).
Admittedly,
the setting of boundaries between functional units by glial networks leaves
open the question of what is controlling the glia. How do glia and neurons
self-organize into functional units? At the present stage of research, one can
only speculate. One explanation could be that molecular feedback mechanisms on
the cellular level play a role (Mitterauer, 2001). Let us take circadian
feedback loops as an example. A circadian system can be made up of one or more
interconnected feedback loops. The constituents of a loop are heterodimeric
proteins such as the BMAL1-CLOCK pair which has domains allowing dimer
formation. These dimers act as positive elements to turn on a gene, such as the
per gene in mice. The encoded PER
protein acts as a negative element in the feedback loop to suppress the
activation of the positive elements (Dunlap, 1998; Hardin, 2000; Lee et al,
2000; Mitterauer, 2000b; Grima et al, 2002). A comparable negative feedback
mechanism could exist between glial cells and neurons (Haydon, 2000;
Mitterauer, 2001). The firing of neurons might stimulate glial cells to release
certain substances, such as neurotransmitters, ions, etc., which subsequently
deactivate the neurons. In this case glia may not only be able to form
functional units with neurons, but they could also set a time limit for the
functional processes (Mitterauer, 2001). The turning off of neurotranmission
can logically be interpreted as a rejection mechanism. Now, what happens when
glial receptors are “chimeric” (intron-containing) due to mutations in splicing
controlling genes?
3.2.2. Loss of the glial boundary-setting function
First
let us suppose that mutations in genes which control the splicing mechanism
will lead to non-splicing of introns. If non-splicing changes the structure of
glial receptors (“chimeric” receptors), then these receptors cannot be occupied
appropriately by their neurotransmitter ligands so that the inactivating
function of synaptic transmission by glial cells is disturbed or lost. It makes
no difference if an excitatory or an inhibitory transmitter system is affected.
The point is that glial receptors cannot be occupied and that, at least
locally, neuronal transmission cannot be interrupted. As a result, neither
excitatory nor inhibitory transmitters are able to act in well defined
spatio-temporal functional units in the brain. The type of imbalance of
transmitter systems will depend on the brain area and the neuronal circuits
affected. A variety of neurotransmitter systems are involved in regulating
information flow via the corticostriatothalamocortical loops, any of which
could be altered in schizophrenia (Wyatt et al, 1995).
Recently,
immunohistochemical findings suggest that in the entorhinal and inferior temporal
cortex of the schizophrenic brain the expression of the GABA (B) receptor is
reduced, raising the possibility that GABA (B) receptor dysfunction is involved
in the pathophysiology of schizophrenia (Mizokami et al, 2002). Huntsman and
coworkers (1998) found that the levels of an alternatively spliced form of GABA
receptor mRNA are reduced by 51% in the brains of schizophrenic patients, which
should have severe consequences for cortical function. Extending these
considerations further, we are not surprised by the fact that alterations in
various transmitter systems have been found in brains of schizophrenic
patients, for instance decreased excitatory synapses in the median temporal
lobe (Harrison and Eastwood, 1998). Therefore, it has been hypothesized that
the antipsychotic action of neuroleptic drugs is due to combined
neurotransmitter effects and not to a primary abnormality of dopamine
neurotransmission alone (Johnstone et al, 1999).
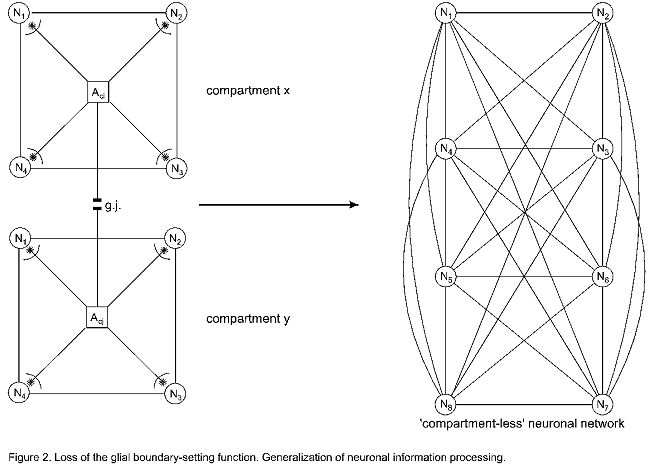
Such a loss of the glial boundary setting function is depicted in Figure
2. The astrocytes (Aci, Acj) have chimeric receptors
(asterisks), so that their processes cannot influence or structure the neuronal
information processing. This genetically determined disturbance results in a
“compartment-less” neuronal network displayed as a graph of eight neurons with
28 connecting lines (according to the formula n/2(n+1)). Such a brain is
“measure-less” informed, i.e. unable to structure the environmental
information. One may argue that the neuronal system is also compartmentalized
per se. As already mentioned, Rall (1995) has developed a compartment model
based on the neuronal system which divides a neuron into a number of
compartments (soma, dendrites, axons). He was able to show that the complete
functions of the neurons could only be demonstrated if their different
functional parts were separated (compartmentalization). According to my view,
there is a qualitative difference between the purely neuronal compartments and
the glia-determined compartments. Neuronal compartments are merely functional
for information processing, whereas glial-neuronal compartments may, in
addition, have an information structuring potency. Depending on the brain areas
affected by mutations in the splicing controlling genes, a “boarder-less”
generalization of functional units (neuronal groups; Edelman, 1981; Costa,
1992; Gupta et al, 2000) results in the inability to reject information on the
behavioral level. Therefore, the boundary between the brain and its environment
is also lost.
The inability to reject non-realizable or “intronic” ideas may result in
delusions and hallucinations, and could explain why schizophrenic patients are
unable to test the reality of their ideas and are at times absolutely convinced
that all which occurs in their brains is real. In other words, the basic flaw
that results in delusions and hallucinations is the loss of the capability to
reject impossibilities concerning the realization of ideas in the environment. It
can be argued that there are diseases caused by genetic changes in
neurotransmitter receptors which still do not produce schizophrenic symptoms,
as for instance in the congenital myasthenic syndromes, which are due to
mutations in acetylcholine receptors (Engel et al, 1998). In the hypothesis now
being presented, schizophrenia will only occur if the following conditions are
met:
1) The
functions of rejection and boundary setting with respect to the environment
that glial cells perform in the brain must be severely disturbed or lost due to
mutations in genes that control splicing. These genetic lesions affect not only
molecular and cellular processes, but also the psychobiological behavior of the
individual.
2) Stressors
(life events, object loss, organic disorders, viral infections, etc.) must
occur to trigger schizophrenic symptoms in the genetically vulnerable
individual (vulnerability-stress model of schizophrenia; McGlashan and Hoffman,
1995).
4. The timeless brain
If one accepts my hypothesis
that the schizophrenic brain has lost the compartmental boundaries within the
brain, it follows that it is also incapable to set boundaries between the Self
and the Non-Selves or the environment. It generalizes, so to speak, to an
infinite universe. Psychiatrists would call this state an inner/outer
confusion. Reality-testing is therefore impossible. But what are the
implications of such a borderless brain for the experience of time?
In a schizophrenic brain time may not really matter. Everything is Now.
This sounds like Barbour´s theory of existence, in which time has no reality.
“Time and motion are nothing more than illusions” (Barbour, 1999). Because I
draw possible parallels between my brain model and Barbour´s theory, a short
pertinent outline of his ideas is necessary.
Barbour´s universe is a geometrically structured, static tableau
composed of Platonic bodies (triangles). Therefore, he termed it “Platonia”.
Specific configurations are covered by a mist, under which a time capsule is
hidden. A time capsule in the sense of a configuration (composed of triangles)
exactly respresents a Now. Every now is a complete, self-contained, timeless,
unchanging universe. The intensity of mist does not vary in time, but it does
vary from position to position. Its intensity at each given point is a measure
of how many configurations corresponding to that point are present. Any
continuous path in Platonia corresponds to a sequence of triangles: they are
the points through which the path passes. At any one point in Platonia many of
them pass through.
Supposing that the brain of a schizophrenic patient embodies a physical
universe without the biological compartmentalization, perhaps he or she is
capable to tap into the many Nows hidden in the structure of the neuronal
network. This ability could also explain how delusions and hallucinations
arise. These basic schizophrenic symptoms could be based on a read out of
configurations or Nows from the physical structure of the “brain tableau”. In
such a static brain structure everything is appropriate and nothing rejected.
Nows do not have intentions. “We are all part of one another, and we are each
just the totality of things from our own viewpoint”(Barbour, 1999). Most
remarkably, this does not only express the view of a great scientist´s world,
but it also describes how the schizophrenic patient experiences his world.
Barbour confesses in his book that he tends to pantheism. The contents of
delusions also support a pantheistic interpretation of the universe.
The revolutionary ideas of Barbour represent a big challenge for a
comprehensive brain theory. If my hypothesis that the glia have a
spatio-temporal boundary-setting function in so called normal brains is
correct, then the loss of this function could open the realm of Platonic
physics as Barbour claims. In Figure 2 a graph is depicted where each point
(neuron) is interconnected , termed as a compartmentless neuronal network. From
a geometrical point of view, this network is composed of many triangels like
Barbour´s Platonia. Considering that the human brain consists of about 1011
neurons (points), the number of possible connecting paths is enormous. There is
in fact a mist over our brains. But what are the clinical correlations of the
spatio-temporal loss of self-boundaries?
5. Clinical correlations
5.1. Interpretation of the loss of spatial
self-boundaries
The
phenomenology of schizophrenia is multi-fold. My hypothesis attempts to explain
the multiple symptoms of schizophrenia in terms of loss of self-boundaries. As
already discussed, mutations in the genes that control splicing may result in a
"borderless generalization of functional units" causing an incapacity
to reject information on the behavioral level. Therefore the patient is unable
to test the reality of his ideas.
We
should first assume that the ability of the human brain to develop new ideas is
nearly unlimited. For example, in the dream state our brains produce scenes
which are not realizable during waking.
Man is only able to fly in his dreams. If a schizophrenic patient states
that he has just been flying, to him this scene produced by his brain has
really taken place, because he cannot differentiate between his inner world and
the outer world. Therefore, everything taking place in the brains of
schizophrenic patients is reality as far as they are concerned. There is a loss
of the capacity to accept information which contradicts the ability to fly and
to reject the delusion as a consequence.
Hallucinations
are caused by the same disorder. If a patient hears the voice of God giving him
commands, then he must obey because he is convinced that the voice belongs to
God. The patient is in no way accessible to the argument that God did not give
the command, because he cannot distinguish between his inner and outer
reality. The loss of self-boundaries is
also evident in the content of delusions. For example, one of my patients is
absolutely convinced that he is simultaneously Ceasar, Napoleon, Churchill and
"Urbi". "Urbi" is a schizophrenic neologism, that is a
novel verbal construction that has a mysterious meaning only to the patient.
If a
patient believes he is God and the Devil in one person, to an observer this
might appear as a split mind and thus schizophrenic. However, I interpret this
delusion to a loss of self-boundaries, since the patient is unable to
distinguish between God and the Devil.
Depending on the brain areas affected, the loss
of the boundary setting function and the corresponding generalization of brain
functions may show up in the motor, affective, or cognitive behavior of the
patient. For instance, catatonic agitation in which a disinhibited discharge of
nearly all motor systems occur, is an expression of motor generalization with
raging and screaming as behavioral components. Here we are probably dealing
with an excess in excitatory transmitter systems. If a catatonic stupor occurs,
which implicates a state of complete motor inhibition, presumably the
inhibitory transmitter systems predominate.
Affective
flattening is regarded as a negative schizophrenic symptom (Dollfus and Petit,
1995). This symptom can also be explained as a loss of boundary setting. The
different affective or emotional qualities cannot be produced within the brain
and the communication of feelings is disturbed as a result. This dysfunction
specifically affects the limbic system. Dysfunction of thought in schizophrenic
patients appears as an incoherence (loss of meaning) of thought processes.
Since the contents cannot be distinguished with respect to their qualitative
independence, the patient is unable to meaningfully sort his thoughts or to reject
meaningless ideas. The loss of spatial boundary function can also appear as
megalomania. For example, one patient said: "I am the universe".
5.2. Interpretation of the loss of temporal
self-boundaries
During extensive
explorations schizophrenic patients confess their convictions that “everything
is as it is”. Typical statements are: “I am living in eternity” or even “I am
eternity”. One of my patients, able to speak with the almighty God, aks him day
by day: when does the world break down? The voice of God is always
disappointing: “no change!” There is obviously a loss of the temporal
boundary-setting function. One could call it a delusion of eternity.
With regard to a pertinent question about time, a patient answered:
“There is nothing which I cannot perceive. Perhaps I am not aware of some
things, but the things are here. Since everything is here and now, evolution is
nonsense.” This schizophrenic sense of existence could be characterized as an
“eternal Now”. In fact, this patient describes his existence similar to
Barbour´s everlasting tableaux that includes everything in the universe at any
given moment. Barbour calls each of these possible still-life configurations a
“Now” as I already did. Again, schizophrenics have absolutely no problem with
the real existence of a delusional idea or the content of a hallucination; they
are “Nows”.
From the perspective of Barbour´s theory, one severe schizophrenic
symptom seems to be especially impressive. It is the so-called catatonic
stupor. Except for the basic cardiac and respiratory functions, the patient
shows no movement or reaction in the sense of a complete psychomotor rigidity.
No communication is possible. Such a patient embodies a “motionless Now”. In
his book “The end of time”, Barbour quotes people with specific types of brain
damage who are unable to see motion where normal people would. His conclusion
is rather surprising: “If the mind can do these things, it may be creating the
impression of motion in undamaged brains.” Similar considerations may also hold
for schizophrenia.
6. Attempting an interdisciplinary definition of the etiology of
schizophrenia
On the molecular
level the non-rejection or non-splicing implies a severe disturbance of
neurotransmission caused by the production of truncated or short-lived proteins
and chimeric receptors such that the glial cells lose their spatio-temporal
boundary-setting function. From a spatial point of view, the loss of glial
barriers may lead to a generalization of neuronal information processing. With
regard to the loss of temporal boundary-setting, the schizophrenic brain may be
interpreted as a timeless universe in line with Barbour´s theory. This may
explain the loss of self-boundaries and the typical symptoms of schizophrenia,
especially delusions and hallucinations (Figure 3). Churchland (2002) also
describes a schizophrenic patient suffering from deep confusion about
Self-/Non-Self boundaries, e.g. responding to a tactile stimulus by claiming
that the sensation belongs to someone else or that it exists somewhere outside
himself. However, Churchland does not offer any explanatory model of the
confusion of Self/Non-Self boundaries.
Now tying all arguments together, schizophrenia could be defined as
follows:
Schizophrenia is a conscious state of a “timeless” misinterpretation of
the Self and the environment due to the inability to set boundaries between the
subsystems (compartments) of the Self within the brain as well as between the
Self and the Non-Selves or the environment. This state is caused by a
non-splicing (non-rejecting) of introns leading to a glia determined
disturbance of neurotransmission and to the loss of the glial spatio-temporal
boundary-setting function. The typical symptoms are delusions and
hallucinations.
7. Conclusion
Up to now, the etiology of
schizophrenia is unknown. The investigation of this psychobiological disorder
is difficult, because of its multifold sympomatology and multifactorial
genesis. Therefore, a theory of schizophrenia should be based on an
interdisciplinary approach.
I started out with the psychiatric hypothesis that the main symptoms of
schizophrenia (delusions and hallucinations) may be caused by a loss of
self-boundaries. First, I defined the concept of the Self as a self-reflecting
system consisting of many subsystems or Selves in the brain. A Self has
intentional programs striving for self-realization in the environment.
Basically, appropriate objects may be accepted, but inappropriate ones must be
rejected. This rejection mecahnism has a boundary-setting function. Therefore,
the loss of self-boundaries may be caused by an ability of the brain to reject
non-realizable programs. This is my basic hypothesis.
At a molecular level, a non-splicing of introns may correspond to a
non-rejection. This would lead to “chimeric” receptors and a significant
disturbance of neurotransmission. Concerning normal brains, I proposed that
glia have a spatio-temporal boundary-setting function grouping neurons into
functional units or compartments. It is assumed that “chimeric” receptors in
glial cells cannot interact properly with their cognate neurotransmitters. The
glia will lose their inhibitory-rejecting function with respect to the
information processing within neuronal networks. The loss of glial barriers may
result in a “borderless” generalization of information processing such that the
structuring of the brain in functional domains is almost completely lost. This
loss of glial boundary-setting could offer an explanation of the loss of
self-boundaries in schizophrenia.
Understood in this sense, the main schizophrenic symptoms can be deduced
both from the loss of spatial self-boundaries and from the loss of temporal
self-boundaries. Most interestingly, the temporal loss of self-boundaries
corresponds to Barbour´s theory of the universe in the sense of a timeless,
everlasting existence or a “Now”. Specifically, many delusions arise from the
conviction that time does not really matter. The subjective universe of those
patients represents an “eternal Now”.
Finally, I attempted to define schizophrenia by tying all my arguments
together towards an interdisciplinary theory. Since my explanatory model of
schizophrenia is testable at least on a molecular level, its further
elaboration towards a comprehensive theory depends on pertinent experimental
results.
Acknowledgements
This
paper is dedicated to John Pellam, the great mediator between sciences. I am
very indepted to Birgitta Kofler-Westergren for preparing the final version of
the paper.
References
Antanitus, D.S., 1998. A
theory of cortical neuron-astrocyte interaction. The
Neuroscientist 4, 154-159.
Baars,
B.J., 1996. Understanding subjectivity: global workspace theory and the
resurrection of the observing self. J.
Consc. Stud. 3, 211-216.
Barbour, J., 1999. The end of time. Weidenfeld
and Nicolson, London.
Carpenter,
W.T., Buchanan, R.W., 1995. Schizophrenia: introduction and
overview. In: Kaplan, H.I., Sadock, B.J.
(Eds.), Comprehensive Textbook
of
Psychiatry VI, vol. 1. Williams and Wilkins, Baltimore, pp.
889-902.
Churchland, P.S., 2002. Self-representation in
nervous systems. Science, 296, 308-310.
Clark,
T.A., Sugnet, C.W., Ares, M., 2002. Genomewide analysis of mRNA processing in
yeast using splicing-specific microarrays. Science, 296, 907-910.
Cooper,
M.S., 1995. Intercellular signalling in neuronal-glial networks. BioSystems 34, 65-85.
Costa,
E., 1992. Building a bridge between neurobiology and mental illness. J. Psych. Res. 26, 449-460.
Cotrina,
M.L., Gao, Q., Lin, J.H., Nedergaard, M., 2001. Expression and function of
astrocytic gap junctions in aging. Brain Res. 901, 55-61.
Damasio,
A.R., 1992. The selfless consciousness. Behav. Brain Sci. 15, 208-209.
Damasio,
A.R., 1994. Descartes´ Error. Grosse H/Putnam, New York.
Damasio,
A.R., 1999. The Feeling of What Happens. Harcourt, New York.
Dollfus, S., Petit, M., 1995. Negative symptoms in
schizophrenia: their evolution during an acute
phase. Schiz. Res. 17,
187-94.
Dunlap,
J.C., 1998. Common threads in eukaryotic circadian systems. Curr. Opin. Gen. Dev. 8,
400-406.
Edelman, G.M., 1981. Group selection as the basis for higher brain
function. In: Schmitt, F.O., Worden, G., Adelman, S., Dennis, G. (Eds.), Organization of the cerebral cortex.
MIT Press, Cambridge.
Edelman,
G., 1992. Bright Air, Brilliant Fire: On the Matter of the Mind. Basic Books,
New York.
Engel, A.G., Ohno, K., Wang, H., Milone, M., Sine, S.M., 1998. Molecular
basis of congenital myasthenic syndromes:
mutations in the acetylcholine
receptor. Neuroscientist 4, 185-194.
Fields,
R.D., Stevens-Graham, B., 2002. New insights into neuron-glia
communication. Science 298, 556-562.
Fisher,
S., Cleveland, S.E., 1968. Body image
and personality. New York,
Dover.
Frith,
C.D., 1979. Consciousness, information processing, and schizophrenia.
Brit.
J. Psych. 134, 225-235.
Frith,
C.D., 1987. The positive and negative symptoms of schizophrenia reflect
impairments in the perception and
initiation of action. Psychol.
Med. 17,
631-648.
Gallo, V., Ghiani, C.A., 2001. Glutamate
receptors in glia: new cells, new
inputs and
new functions. Trends Pharm. Sci. 21, 252-258.
Gallo, V., Giovanni, C., Suergin, R., Levi, G.,
1989. Expression of
excitatory amino acid
receptors by cerebellar cells of the type-2 astrocyte
cell linkage. J. Neurochem. 52, 1-9.
Gray,
J.A., 1991. The neuropsychology of schizophrenia. Behav.
Brain
Sci. 14, 1-84.
Grima, B., Lamouroux, A., Chelot, E., et al, 2002. The
F_box protein Sbinb
controls the levels of clock proteins
Period and Timeless. Nature 420,
178-182.
Grosche,
J., Moller, M.V., Verkhratsky, A. et al, 1999. Microdomains
for
neuron-glia interaction: parallel fiber
signalling to Bergman glial cells. Nat.
Neurosci. 2, 139-143.
Günther,
G., 1962. Cybernetic ontology and transjunctional operations. In:
Yorvits, M.C., Jacobi, G.T., Goldstein,
G.D. (Eds.), Self-organizing
Systems. Washington, Spartan Books.
Gupta, A., Wang, Y., Markram, H., 2000. Organizing principles
for a
diversity of GABAergic interneurons and
synapses in the neocortex. Science
287, 273-278.
Hall,
J.C., 1995. Tripping along the trail to the molecular mechanisms of
biological clocks. Trends Neurosci. 18,
230-240.
Hardin,
P.E., 2000. From biological clock to biological rhythms. Gen.
Biol.
1 (4): reviews, 1023:1-1023:5.
Harrison,
P.J., Eastwood, S.L., 1998. Prefrontal involvement of excitatory
neurons in medial temporal lobe in
schizophrenia. The Lancet 352,
1669-
1673.
Haydon,
P.G., 2000. Neuroglial networks: neurons and glia talk to each other.
Curr.
Biol. 10, R712-R714.
Hemsley,
D.R., 1982. Cognitive impairment in schizophrenia., In: Burton, A.
(Ed.), The pathology and psychology of cognition. Methuen.
Hemsley,
D.R., 1987. Hallucinations. Unintended or expected? Behav.
Brain
Sci. 10, 532-533.
Heyman,
I., Murray, R.M., 1992. Schizophrenia and neurodevelopment. J
R Coll
Physicians Lond. Apr;
26(2), 143-146.
Huntsman,
M.M., Tran, B.V., Potkin, S.G., Brunney, W.E., Jones, E.G.,
1998. Altered ratios of alternatively
spliced long and short gamma 2 subunit
mRNAs of the gamma-amino butyrate type A
receptor in prefrontal cortex of
schizophrenics. Proc. Natl. Acad. Sci. USA
95, 15066-15071.
Iberall,
A.S., McCulloch, W.S., 1969. The organizing principle of complex
living systems. Transactions of the ASME,
6, 290-294.
Johnstone,
E.C., Humphreys, M.S., Lang, F.H., Lawrie, S.M., Sandler, R.,
1999. Schizophrenia. University
Press, Cambridge.
Kilmer,
W.L., McCulloch, W.S., Blum, J., 1969. A model of the vertebrate
central command system. Int. J. Man-Mach. Stud. 1,
279- 309.
Kimelberg,
H.K., Jalonen, T.O., Aoki, C., McCarthy, K., 1998. Transmitter
receptor and uptake systems in astrocytes
and their relation to behavior. In:
Laming, P.R., Sykova, E., Reichenbach, A.,
et al (Eds.), Glial cells: their role
in behaviour. Cambridge,
University Press, pp. 107-129.
Lee, K., Loros, J.J., Dunlap, J.C., 2000. Interconnected
feedback loops in
the neurospora circadian
system. Science 289,
107-110.
Lewis, D.A., 2000. Is there a neuropathology of schizophrenia? Recent
findings converge on altered
thalamic-prefrontal cortical connectivity. The
Neuroscientist 6, 208-218.
Liu,
K., Bergson, C., Levenson, R., Schmauss, C., 1994. On the origin of
mRNA encoding the truncated dopamine
D3-type receptor D3nf and detection
of D3nf-like immunoreactivity in human
brain. J. Biol.
Chem. 269,
29220-29226.
Martin,
D.L., 1995. The role of glia in the inactivation of neurotransmitters. In:
Kettenmann, H.,
Ransom, B.R. (Eds.), Neuroglia. Oxford University
Press, New York, pp. 732-745.
Maynard, T.M., Sikich, L., Lieberman, J.A., et al, 2001. Neural
development,
cell-cell signalling, and the “two-hit”
hypothesis of schizophrenia.
Schiz. Bull., 27, 457-476.
McGlashan,
T.H., Hoffman, R.E., 1995. Schizophrenia: Psychodynamic to
neurodynamic theories. In: Kaplan, H.T., Sadock, B.J. (Eds.),
Comprehensive Textbook of Psychiatry VI, vol. 1, Williams and Wilkins,
Baltimore, pp. 957-968).
Mennerick, S., Benz, A., Zorumski, C.F., 1996. Components
of glial responses
to exogenous and synaptic glutamate in rat
hippocampal microcultures. J.
Neurosci. 16, 55-64.
Mitterauer, B., 1998. An interdisciplinary approach
towards a theory of
Consciousness. BioSystems, 45, 99-121.
Mitterauer,
B., 2000 a. Some principles for conscious robots. J.
Intell.
Sys. 10, 27-56.
Mitterauer,
B., 2000 b. Clock genes, feedback loops and their possible role in
the etiology of bipolar mood disorders: an
integrative model. Medical
Hypotheses 55(2),
155-159.
Mitterauer, B., 2001. The loss of ego boundaries in
schizophrenia: a
neuromolecular hypothesis. Medical Hypotheses 56
(5), 614-621.
Mitterauer, B., Leitgeb, H., Reitboeck, H.,
1996. The neuro-glial
synchronization
hypothesis. Rec. Res. Devel. Biol.
Cybernet. 1, 137-155.
Mitterauer, B., Garvin, A., Dirnhofer, R., 2000. The
sudden infant death
syndrom (SIDS): a
neuromolecular hypothesis. The Neuroscientist 6,
154-158.
Mizukami, K., Ishikawa, M., Hidaka, S., et al,
2002. Immunohistochemical
localization
of GABA(B) receptor in the entorhinal cortex and inferior
temporal
cortex of schizophrenic brain. Progr.
Neuropsychopharmacol. Biol. Psych. 26,
393-396.
Murphy, T.H., Blatter, L.A.,
Wier, W.G., Baraban, J.M., 1993. Rapid
communication between neurons and astrocytes in primary cortical
cultures. J.
Neurosci. 13, 2672-2679.
Newman, J., 1997. Putting the puzzle together,
Part I: towards a general theory
of
the neural correlates of consciousness. J. Consc. Stud. 4,
47-66.
Newman, E.A., Zahs, K.R., 1997. Calcium waves
in retinal glial cells. Science
275,
844-846.
Nishiyama, H., Knopfel, T., Endo, S., et al, 2002.
Glial protein S100B
modulates long-term neuronal synaptic plasticity. Proc. Nat.
Acad.
Sci. USA 99, 4037-4042.
Oliet, S.H., Piet, R., Poulain, D.A., 2001. Control of glutamate
clearance and
synaptic efficacy by glial coverage of
neurons. Science 292, 923-926.
Parri,
H.R., Gould, T.M., Crunelli, V., 2001. Spontaneous astrocytic Ca2+
oscillations in situ drive NMDAR-mediated
neuronal excitation. Nat.
Neurosci. 4, 803-812.
Rall,
W., 1995. Theoretical significance of dendritic trees for neuronal input-
output relations. In: Segev, I., Rinzel,
J., Shepherd, G.M. (Eds.), The
theoretical foundation of dendritic
function. Cambridge, The MIT Press, pp.
122-146.
Reichenbach, A., Skatchkow, S.N., Reichelt, W., 1998. The
retina as a
model of glial function in the brain. In:
Lamig, P.R., Sykova, E., Reichenbach,
A., Hatton, G.I., Bauer, H. (Eds.), Glial cells: their role in behavior.
University
Press, Cambridge, pp. 63-82.
Scheibl, M.E., Scheibl, A.B., 1968. The brainstem reticular core
– an
integrative matrix. In: Mesarovic, M.D. (Ed.), Systems theory and
biology.
Springer, New York, pp. 261-285.
Schmauss,
C., 1996. Enhanced cleavage of an atypical intron of dopamine D3-
receptor pre-mRNA in chronic schizophrenia.
J. Neurosci. 16,
7902-7909.
Shastry,
B.S., 2002. Schizophrenia: A genetic perspective. Int.
J.
Mol. Med. 9, 207-212.
Sims,
A., 1991. An overview of the psychopathology of perception: first rank
symptoms as a localizing sign in
schizophrenia. Psychopathol. 24,
369-374.
Smit,
A.B., Syed, N.I., Schaap, D., et al, 2001. A glial-derived acetylcholine-
binding protein that modulates synaptic
transmission. Nature 411, 261-268.
Smolin,
L., 1997. The life of the cosmos. Oxford University Press, New York.
Strahonja-Packard,
A., Sanderson, M.J., 1999. Intercellular Ca2+ waves induce
temporally and spatially distinct
intracellular Ca2+ oscillations in glia. Glia,
28,
97-113.
Steindler, D.A., 1993. Glial boundaries in the developing nervous
system. Ann.
Rev. Neurosci. 16,
445-470.
Steriade, M., 1996. Arousal:
revisiting the reticular activating system. Science
272, 225-226.
Sykova, E., Hansson, E., Rönnbäck, L., Nicholson, C.,
1998. Glial
regulation of the neuronal microenvironment.
In: Laming, P.R., Sykova, E.,
Reichenbach, A., Hatton, G.I., Bauer, H.
(Eds.), Glial cells: their role in
behaviour. University
Press, Cambridge, pp. 130-163.
Teichberg, V.I., 1991. Glial
glutamate receptors: likely actors in brain
signaling. FASEB Journal 5, 3086-3091.
Ullian, E.M., Sapperstein, S.K.,
Christopherson, K.S., Barres, B.A., 2001.
Control of synapse number by glia. Science 291, 657-660.
Varon,
S.S., Somjen, G.G., 1979. Neuron-glia interactions. Neurosci.
Res.
Progr. Bull. 17, 1-239.
Werner,
G., Black, F.O., Cornes, C., Larkin, A., Steinhauer, S., 1982.
Anomalies of
neuro-sensory functions and representational world in
schizophrenics. In: Msokir, E., Handin, I.
(Eds.), Biological markers in
psychiatry and neurology, Oxford,
Pergamon Press.
Wyatt,
R.J., Kirch, D.G., Egan, M.F., 1995. Schizophrenia: Neurochemical,
viral, and immunological studies. In:
Kaplan, H.T. and Sadock, B.J. (Eds.),
Comprehensive
Textbook of Psychiatry VI, vol.
1. Williams and Wilkins,
Baltimore, pp. 927-942.
Table 1. A subject tries to realize its intentional program,
depicted as intentional values iv (1, 3, 2, 4), in four steps (step 1…4). Two
objects, with their respective object values ov (1, 2), are found in the environment.
In the first step, the detected object ov (1) can be accepted. In the second
step, the intended value iv (3) rejects both objects ov (1, 2). In the third
step, ov (2) is accepted. In the fourth step, finally, iv (4) rejects both
object values ov (2, 2) (Mitterauer, 2000a).
Figure 1. Glial boundary-setting function in its
interaction with the neuronal system:
An astrocyte (Aci) is connected with four neurons (N1…N4)
via processes building a glial-neuronal compartment (x). Only two processes are
activated influencing the neurotransmission in the synapses (sy) of N2
and N3 (thick lines). In the second compartment (y), the astrocyte
(Acj) activates three neurons (N1, N2, N4).
These two compartments are connected via a gap junction (g.j.). Dashed lines
outline the neuronal network determined by the activated neurons in both
compartments.
Figure 2. Loss of glial boundary-setting function and
generalization of neuronal information processing:
In the glial-neuronal compartments (x, y) the astrocytes (Aci, j)
are unable to activate any of the neurons (N1…N4). They
cannot influence the neurotransmission in the synapses (asterisks). This loss
of glial boundary-setting (as depicted in Fig. 1) results in a
‘compartmentless’ neuronal network where all neurons are interconnected.
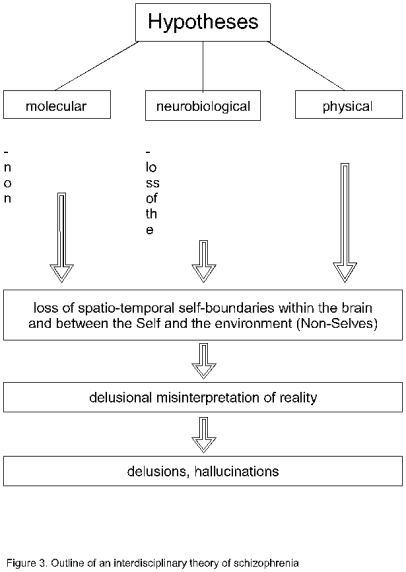
Figure 3.
Outline of an interdisciplinary theory of schizophrenia.
On the molecular level, a non-splicing of introns results in truncated
proteins or “chimeric” receptors. On the cellular level, a loss of the glial spatio-temporal
boundary-setting function may cause a disturbance of neurotransmission. In
generalizing the neuronal information processing the brain functions as a
compartmentless neuronal network (see Figure 2). From a physical point of view,
such a brain can also be interpreted as a timeless brain. Therefore,
schizophrenia may be caused by the loss of spatio-temporal self-boundaries
within the brain and between the Self and the environment (Non-Selves). This
disorder leads to a delusional misinterpretation of reality. Typical symptoms
are delusions and hallucinations.
Dr. Bernhard
Mitterauer is Professor of Neuropsychiatry at the University of Salzburg's
Institute of Forensic Neuropsychiatry. He recived his M.D. from the
University of Graz and eight years later received his academic degree in
Neuropsychiatry and Psychoanalysis. Dr. Mitterauer studied Philosophy with
Gotthard Gunther, the famous Philosopher of Cybernetics, in Hamburg. He
developed a close friendship and intensive scientific collaboration with
Gunther, whose philosophy has influenced Dr. Mitterauer's work up to this day.
In 1984 Dr. Mitterauer was appointed Professor of Neuropsychiatry at the
University of Graz and he has been serving as a Professor and Head of Forensic
Neuropsychiatry at the University of Salzburg since 1989. Concurrently with his practical work as a
Neuropsychiatrist, Dr. Mitterauer has been involved in interdisciplinary
research in Biocybernetics since the beginning of his professional career. In
the 1970's he published basic research studies on emotion, depression,
narcissism and self-observation. Notably, in 1981 he earned the Eiselberg Award
for his already internationally acknowledged research on suicide. During the
1980's he published numerous studies dealing with a new "dialectic"
psychopathology. His book "Architectonics, Metaphysics of
Feasibility" deals with a future-oriented interpretation of technical
activities, especially the development of robots. Dr. Mitterauer decisively
advanced the methodology of the assessment of criminal offenders and has
published numerous pertinent basic research studies. He is the founder of the
Gotthard Gunther Archives for the research and publication of the posthumous
works of Gunther at the University of Salzburg.
[ BWW Society Home Page ]
© 2008 The Bibliotheque: World Wide Society