Ways Out of the BSE Crisis - Alternative Solutions
by Professor Dr.Dr.h.c. Randolph Riemschneider, Life B.Fel.[1]
Instituto Central de Quimica da Universidade Federal de Santa
Maria (UFSM), Santa Maria, RS, Brazil
Institute of Biochemistry, Free University of Berlin (FUB), Germany
[Editor’s Note: This paper is presented as Part I of a two-part series]
Keywords: Animal extracts: synthetic, cell culture based - plant extracts: yeast, cereal, glycoprotein based - chemicytorrhysis - activation of cell metabolism, fermentation and respiration increasing factor; (WARBURG tests), ASO- and ASD-inhibition - applications in cosmetics, in medicine, as animal food additive, as food drug - whitening effect - proteinfree extracts - trade marks – BSE[2]
SUMMARY
The following experimentally secured production processes and their application to migrate the consequences of the BSE crisis described in I – VI have been developed by the author:
I Aqueous synthetic organ extracts (1)
II Organ extracts, cell culture based (2a)
III Cell line organ material beats BSE risk in animal extracts (2b)
IV Plant material substitutes organ extracts: yeast-, cereal-, glycoprotein-based
IV 1 Thallophytic and bryophytic chemicytorrhysates (5a-c)
IV 2 Biophysically derived ascomycetes and schizomycetes preparations (7a-c)
IV 3 Fermentation-promoting cell preparations on yeast basis (8a-d)
IV 4 Cereal based extracts: Vegetal placenta extracts (11a-d)
IV 5 Cell wall glycoproteins: Collaplant PO (9)
V SPECIAL PART 1-6
VI APPENDIX 1-3
Some of these patent applications opened new ways for the ortho-molecular medicine[3], i.e. (1, 2, 14); cf also (15,16,17).
I.
Preparation and application of aqueous synthetic organ extracts:
Patent specification EP 0 552 516 B1, dated December 2, 1992 (inventor: the author), 38 pages, 25 claims (1).
The invention relates to aqueous synthetic organ extracts which show at least the efficiancy of a corresponding natural organ extract to be used for cosmetic and medical preparations.
A few examples of the organ extracts addressed in this patent specification are
- a synthetic placenta extract and its cosmetic action
- a combination of a synthetic serum/placenta extract;
- a synthetic spleen extract and detection of its metabolic activity;
- a synthetic thymus extract and studies of toxicity;
- a synthetic serum extract and its effect on damaged fibroblasts;
- synthetic collagen extract and a synthetic connective tissue extract with examination for skin care characteristics and increased cell activity;
- synthetic organ extracts without preservation (for cell culture trials such as clone tests, etc.):
- synthetic proteinfree organ extracts
Gastro explantat under the influence of synthetically produced organ extracts without preservating agent, prepared according to example 4

In (1), the author has summarised the experience with synthetic animal extracts and vegetable extracts collected over many years which were exploited starting in 1960. Patent protection was not sought until the BSE crisis. A synthetic proteinfree organ extract was produced at industrial scale and put to clinical use as early as 15 years before the BSE crisis: PROJECT XXIII in (20).
This essay also shows which of the patented results of (1) as well as (2b, 5a.b, 7a-c, 8a-d, 19) are already being put to use by the industry; see also conclusion (to be found right after VI 4).
II.
Preparation and application of organ extracts on cell culture basis starting from cell cultures free of BSE:
Patent specifications: DE 196 24 476 C2 dated June 19, 1996 (inventor: the author), ms 54 p, 36 claims, 12 figures (2a) – analogue CH 692 408 A5
Title and preamble of the patent specification: Water-soluble organ extracts with an improved biochemical degree of action, and methods for preparing and using the same - preamble in Plate 1 (next page).
In this patent specification (2a) there are three tables:
- Table 1: summary of animal cell lines that may be used for the invention
- Table 2: methods of dermatological compatibility applied and toxicology;
- Table 3: eight methods, used to test the action of the organ extracts
There are 11 examples, some of which will be mentioned here:
- Preparation of a bovine kidney extract from the MDBK cell line;
- Combination of this extract with a corresponding synthetic bovine kidney extract;
- Amnion extracts from the WISH cell line and cosmetic applications;
- Preparation of a placenta extract by combining an extract from the JAR cell line with a synthetic placenta extract;
- Preparation of an aqueous thymus extract from the P1-1A3 cell line combined with a synthetic one plus cosmetic applications;
- Preparation of an aqueous blood extract from the B95.8 cell line; with combinations and testing for the growth of damaged fibroblasts and the growth of tadpoles (Xenopus laevis);
- Preparation of a plant extract of a sycamore cell culture for use as a collagen substitute (comparison of its cosmetic formulations with a collagen preparation);
- preparation of a yeast extract of partially dehydrated sacchromyces cells with applications;
- Various extract combinations with respiration enhancement tests according to WARBURG with radical scavenger tests [example 10: HXX systems = hyaluronic acid, xanthine, xanthinoxidase (2a)]
Plate 1: Preamble to patent DE 196 24 476 C2 (2a) [document]

III
Cell line based organ material beats BSE risk in animal organ extracts (2b).
The author compares the properties of SWINE organ extract - prepared from BSE-free cell lines - with the properties of SWINE extract received from fresh organ glands and - with those of synthetic SWINE extract:
Plate 2: The three manufacturing methods, Plate 3: Activity data of the three SWINE extracts.
In the 60ies, the author prepared for the first time natural SWINE
placenta extracts for application in cosmetics and medicine (3). These were
used in large scale in
In December 2000 the import of any bovine material was stopped by Asian countries. Japanese customers changed to natural SWINE placenta extracts – in consequence of the author’s publication: of 2001, entitled “PORCINE based organ extracts guarantee full substitution of BOVINE extracts” (4b): The results, collected already in the 60ies and 70ies, are summarized in the following Plates 3a and 3b.
Preparations based on natural SWINE organ material can be used in cosmetics and medicine as long as no prions are to be discovered in such material; but in case – the way out then would be to work with synthetic or cell line material.
Plate 2: Three Manufacturing Methods for Organ Extracts: I, II, and III
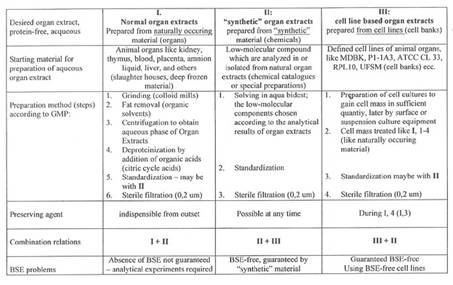
Plate 3: Activity tests of SWINE Placenta extracts prepared according to methods III, III+II and III+I (plate 2)
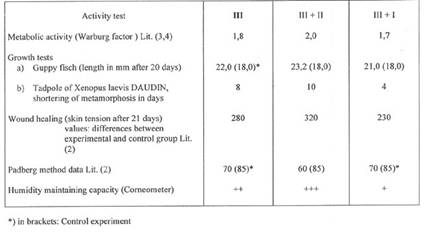
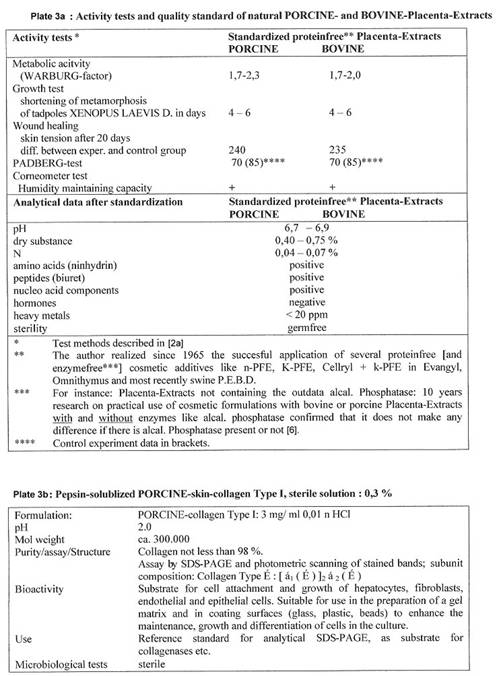
For
more information on proteinfree porcine extacts see also Section VI 4,
re b)
IV
Replacement of organ extracts by suitable plant materials such as those on the basis of yeasts IV1, cereals IV 2, and glycoproteins IV 3
The author had worked on this topic long before the outbreak of BSE and the effects of the crisis, namely under the aspect of developing "plant extracts enhancing the metabolism". This work took on added significance under the influence of the BSE crisis as shown by the patent applications with different uses as discussed below from 1985 onwards.
Basis: Yeasts "transformed"
The starting point for the "Yeast Patents" discussed below under IV 1-3 was the search for a potency inhibition factor (P-I factor):
This is because we were able to show in orientation experiments starting in 1937 (21a,b), and then in statistically supported serial experiments with mice from 1946/47 (22) [1000♂ : 4000♀ each time] that yeast in the drinking water inhibits the potency of male mice. This resulted in two different approaches: a) The development of an aphrodisiac testing method and b) the search for the P-I factor mentioned above. Even though the latter did not result in the isolation of such a factor so far, it led instead to the discovery and development of novel yeast preparations with a highly active cell metabolism, detectable, for example, with the aid of the WARBURG method by activation of fermentation and respiration of suitable substrates. Plant cells have stronger cell walls than animal cells so that less stringent conditions are generally used to obtain animal extracts than in the case of plant extracts. We failed to take this into account in our yeast cell extraction experiments with Saccharomyces in 1946 belonging to group b) and therefore arrived at surprising results at the time, which, in subsequent years, led to the results and applications described in patents: IV 1-3 and SPECIAL PART.
IV 1
Process for the production of thallophytic or bryophytic cytorrhysates, so produced chemicytorrhysates and their use as additional foodstuffs.
EP 0 208 805 A1 vom 19.07.1985 (inventor: author) 34 p (5a), 21 claims
DE 3 402 169 C2 vom 23.01.1984, 34 p und Japan 9807/85 (5b) sowie Teilungen des DE P (5c)
Abstract:
The process entails suitable intact cells undergoing chemicytorrhytic treatment in such a way that they are initially mixed at 0° to 40° C with a water-soluble alkali metal or alkaline earth metal salt with liquefaction and then heated to a temperature above 40°C but below the pasteurisation temperature and finally cooled. These chemicytorrhysate biomasses are suitable as nutrient supplementation for animal feed and drinking water, as plant growth promoter, as cosmetic ingredient for activating skin metabolism and as aphrodisiac for increasing potency. 2 figures, 17 fotographs.
A few examples of the cytorrhysates described in this patent specification are:
Chemicytorrhysates on the basis of wine yeast, baker's yeast and bottom yeast (8 : 90 : 2) show a remarkable plant growth promoter effect as presented in 17 photographs in the patent specification: (5a,c); cf. also ref (18).
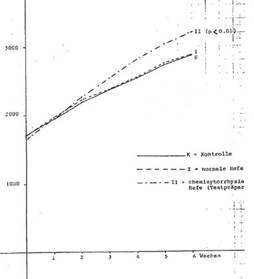
Chemicytorrhysate based on Saccharomyces cerevisiae reduces the consumption of feed: growth effectiveness (rabbits): Tab 1 and 2, Diag 1 (next page)
As mentioned above the sexuality of male mice can be inhibited by yeast (statistically guaranteed). Under the influence of one of the cytorrhyzed yeast mentioned in VI 3 (not revealed here) 10-20% compensation of the inhibition was observed in the following serial experiments, each time with 5000 mice (1000 males with inhibited sexuality).
Tab 1: Growth experiments on rabbits ♂ (body weights in g)
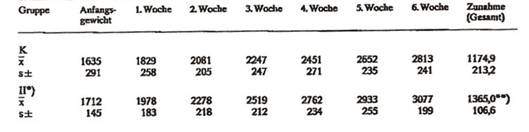
Tab 2: Growth experiments on rabbits ♀ (body weights in g)

Diagram 1: Growth development of female rabbits over 6 weeks
According to the invention EP 0 208 805 A1 (5a) as starting material we used a) bryophytic and b) thallophtic cells - to a) belong musci (mosses), hepaticae (liverworts), and anthroceretes (horned l.); to b) belong algae and fungi, latter classified in ascomycetes sac fungi (saccharomyces, aspergillus, neurospora), basidiomycetes club fungi, and fungi imperfecti; more about the ascomycetes taken into consideration in the patents ref.(5,7,8) you find in section VI 3.
The German patent application (5a) was divided, and the German patent "Aphrodisiac for increasing potency" DE 3 448 223.7 C2 dated Jan 23, 1984 derived from the former (5c).
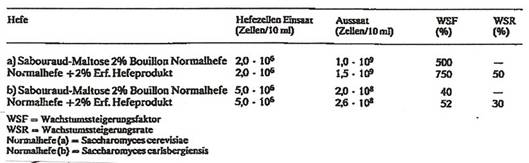
Preparations on the basis of Saccharomyces cerevisiae stimulate the growth of bacteria such as Lactobacillus and Bifidus species as well as of regular yeasts. Experimental data in Tab 3:
Thanks to the chemicytorrhysis achieved, the yeast cells became smaller and their cell walls thicker. It is important that only partial dehydration takes place. It is well known that part of the cell water may be removed from plant cells under suitable conditions. In many cases, this removal is accompanied by a reduction of volume with shrivelling. Most ascomycetes species have a highly elastic cell membrane so that the cells become smaller when water is removed. This process may also be called "de-swelling". Under certain conditions, however, chemical changes regarding the cell components are initiated when water is removed which will lead to the release or formation of activity factors. In turn, these have a catalysing effect or an effect that may be explained in another way on systems subject to anaerobic glucose degradation. The chemical changes of the cell components may be controlled and become more pronounced when influences of heat, osmosis or and/or certain chemical substances and/or other physical influences occur at the same time. In that respect, one could also speak of "chemiosmotic thermoplasmolysis".
IV 2
Biophysically derived ascomycetes, schizomycetes and yeast preparation contained the food and growth material of plants, and their use in treating the skin and in probiotic activation.
DE 3 711 054 C 2 vom 02.04.1987, (inventor: author), 8p (7a)
EP 0 286 033 A2 from 31.03.1988 (inventor: author), 13p, 30 claims, 1 fig., 6 examples (7b)
Corresponding patent applications in: USSR, Polen, Ungarn, CSSR, Dänemark, Australien, Portugal (7a)
Leveduras biofisicamente transformadas: Brasilian Patent application P 1 860 3405 from 31.03.1988 (inventor: author), 15 claims (7c)
Plate 4: 13 Patent claims to 3711054 C 2 from 2.4.1987 (7a) [document]
1. Biophysikalisch derivatisiertes Hefepräparat mit atmungssteigernder Aktivität, dadurch gekennzeichnet, daß der Atmungssteigeurngsfaktor bei 37°C mindestens 2,0 beträgt, bezogen auf das standardisierte Rattenleberhomogenat und ausgedrückt als das vom Homogenat zusätzlich veratmete Sauerstoffvolumen unter ansonsten gleichen Versuchsbedingungen.
2. Hefepräparat nach Anspruch 1, dadurch gekennzeichnet, daß der Atmungssteigerungsfaktor bei 37°C einen Wert von 2,0 bis 6,0 und vorzugsweise einen Wert von 2,5 bis 4,5 aufweist.
3. Hefepräparat nach Anspruch 1, dadurch gekennzeichnet, daß es außerdem einen L-Amino-säureoxidase-Hemmfaktor enthält, meßbar im WARBURG-Versuch an einer standardisierten Testlösung mit dem Enzym und Phenylalanin als L-Aminosäure und ausgedrückt als Verhältnis des Sauerstoffverbrauchs mit und ohne Hefepräparat.
4. Hefepräparat nach Anspruch 3, dadurch gekennzeichet, daß der L-Aminosäureoxidase-Hemmfaktor einen Wert im Bereich von 20 bis 80 % und vorzugsweise einen Wert im Bereich von 30 bis 60% hat.
5. Verfahren zur Herstellung eines biophysikalisch derivatisierten Hefepräparates mit atmungssteigernder Aktivität des Anspruchs 1, dadurch gekennzeichnet, daß eine Ausgangshefe durch Einwirkung von Laserstrahlung, Gefriertrocknung, Ultraschallbehandlung, kurzzeitige Druckbeaufschlagung und/oder Osmolyse solange behandelt wird, daß es zu einer Verkleinerung der Hefezellen auf das 0,5fache oder weniger kommt, während die Zellwände stärker werden, und die Suspension der kontrahierten Hefezellen einer kurzzeitigen intensiven Wärmeschockbehandlung unterzogen wird, bis durch Gärung gebildete Gase entweichen.
6. Verfahren nach Anspruch 5, dadurch gekennzeichnet, daß der Ausgangshefe ein flüssiger Träger zugesetzt wird.
7. Verfahren nach einem der Ansprüche 5 bis 6, dadurch gekennzeichnet, daß man die Hefezellen nicht länger als 25 Min. bei einer Temperatur über 40°C wärmebehandelt.
8. Verfahren nach einem der Ansprüche 5 bis 7, dadurch gekennzeichnet, daß die Wärme-behandlung direkt mit einem im Gegenstrom zugeleiteten Heißgas bewirkt wird.
9. Verfahren nach einem der Ansprüche 5 bis 8, dadurch gekennzeichnet, daß die biophysikalische Zellverkleinerung gleichzeitig mit der kurzzeitigen intensiven Wärmeeinwirkung ausgeführt wird.
10. Verfahren nach einem der Ansprüche 5 bis 9, dadurch gekennzeichnet, daß als flüssiger Träger eine wäßrige Lösung oder Suspension zugesetzt wird, die enthält 1) - 5):
1) 0,1–0,5% biophysikalisch derivatisierte Hefe, die entweder aus einer Hefe, die als Ausgangsmaterial dient und/oder aus einer anderen Hefe erhalten wurde, und 0,02-0,4% Chondriosomen-Extrakt,
2) 0,3-5 % Celluloseglykolat, Carrageen oder Traganth
3) 0,01-0,3 % vergärbare Mono- und/oder Disaccharide,
4) als Konservierungsmittel Nipagine und Salze in ausreichender Konzentration,
5) Spurenelemente wie Mn, Co, Zn, Mg, vorzugsweise als Sulfate oder Glukonate.
11. Verfahren nach einem der Ansprüche 5 bis 10, dadurch gekennzeichnet, daß als Ausgangshefe ein Stamm aus der Familie der Saccharomycetaceae eingesetzt wird.
12. Verfahren nach einem der Ansprüche 5 bis 11, dadurch gekennzeichnet, daß eine Trockenhefe mit einem HTS-Gehalt von 95–98% zusammen mit der 2 – 4fachen Menge des flüssigen Trägers behandelt wird.
13. Verwendung der biophysikalisch derivatisierten Hefe mit atmungssteigernder Aktivität nach einem der Ansprüche 1 bis 12 in einer Menge bis zu 1 Gew.-% als Zusatz in einem herkömmlichen Futter.
Abstract:
A biophysically derived yeast preparation is proposed which
- stimulates respiration in liver homogenate (A)
- contains an amino acid oxidase inhibiting factor (B),
- exhibits excellent cicatrization properties (C),
- RES-stimulation action (D).
A,B,C,D correspond to SPECIAL PART V 1 - V 4
Ad A): The yeast preparations subjected to biophysical derivatisation show a respiration-enhancing effect in WARBURG tests (Tab 4), just as the yeast cell preparations described under IV 3.
Tab 4: WARBURG tests
|
No. |
Sample |
Respiration-enhancing factor |
Increase in percent (%) |
|
1 |
Overall solution |
4.4 |
340 |
|
2 |
Centrifuge supernatant |
4.0 |
300 |
|
3 |
Centrifuge residue |
2.8 |
180 |
|
4 |
Starting material |
1.0 |
- |
Experimental details in SPECIAL PART V 1.
Ad (B) In addition to the respiration-enhancing effect, most of the yeasts of the invention show an amino acid oxidase-inhibiting effect, namely amino acid oxidases (1.4.3.2 or 3, respectively), monoamino oxidases (1.4.3.4) and diaminooxidases (1.4.3.6). As a result of this inhibition, more amino acids remain intact (their C portion is not "burnt") and they are available for building up protein (faster growth). The organisms satisfy their energy requirements from other sources. For experimental details, please refer to the SPECIAL PART V 2.
Ad (C) and (D): Details in SPECIAL PART V 3 und V 4
IV 3
Fermentation-increasing cell preparations on plant cell basis
DE 4 042 157 C 2 from 28.12.1990 (inventor: author) 14p, 14 claims, 8 figures, (8a)
Swiss Patent 684 274 A 5 from 24.12.1991 (inventor: author) 18p, 30 claims, 8 figures (8b). - Japan 90058/92 (8d)
|
Fermentation-increasing cell preparations on plant basis, methods of preparation and the use thereof
The fermentation-increasing cell preparation on plant basis consisting of partially dehydrated plant cells which are no longer capable of reproduction increases the anaerobic glucose degradation by at least 50 %. This cell preparation is used especially for preparing food and feed substances for increased performance and for the ATP formation in yeast suspensions containing adenosine and phosphate, for the preparation of cures to increase fertility and potency and for the preparation of cosmetics.
|
|
Diag. 2 Influence on fermentation
(For details regarding the compositions of the solutions, please refer to SPECIAL PART V 5,2)
VR-China 91 10 5974.1 vom 23.08.1991 (inventor: author), 29p
30 claims, 8 figures (8c); claims in SPECIAL PART V 5: Plate 7a,b

The probiotic "Yeast-based animal food additive" is based on the experience from the three patents discussed above (5,7,8). It has turned out to be especially useful during the BSE crisis and was tested under the designations H 2000, Y 2000, S 2000, Proval and others in many countries (10): Plate 5 and (24-27)
Plate 5: Animal food additive H 2000, Y 2000, S 2000 (10)
H 2000 is a new biological plant-based <<animal food additive>>, tested during the last ten years in large scale on chicken, pigs, sheep, calves, rabbits, dogs, etc. in many countries like Brazil, Japan, USSR (24), Poland (25), Hungary (26), China (27), Switzerland and Germany under the names:
- Proval (Pro from probiotic, val from value) (Proval sometimes Socoproval)
- H 2000 (H from Hefe)
- Y (Y from yeast)
- S 2000 (S from Saccaromyces cerevisiae).
The decisive viewpoint for the development of H 2000 was to use vegetal starting material.
Starting from yeast like Saccaromyces – applying several combined physical influences in the manufacturing process, we came to new << yeast cell-preparations >>, not anymore reproductive and not anymore fermentating, but activating the yeast-fermentation of glucose and activating the cell-respiration in a high degree: more scientifically spoken: activating anaerobic and aerobic reactions of the cell-metabolisms in general. H 2000 is really something new: H 2000 is not to be confused with the normal food-yeast which is used since long time as donator for proteins and B-vitamins.
H 2000 is added to animal food in very low concentration of 0,1%-0,2%. Such low concentrations are sufficient to activate the cell metabolism in different ways – so increasing the effectivness of foodstuff. The patented manufacturing process does not imply any chemistry, no organic solvents are necessary. H 2000 shows no toxicity. One not can observe any mortality determination DL50 mouse, using 15gr H 2000 pro kg mouse.
In the mentioned filed-experiments with thousands and thousands of chicken, hundreds of pigs, cattle and other animals – never was the observed mortality higher than in the control experiments, if any.
Activation of cell-metabolism by H 2000:
The cell-metabolism activation of H 2000 can be demonstrated: anaerobic by increased fermentation (I), aerobic by increased cell-respiration (II), for instance by WARBURG-method.
I: Manometric measurement of increased CO2-production of fermenting yeast under H 2000 influence or
II: Manometric measurements of increased O2 – consumption of liver homogenate under H 2000 influence.
The activation of the anaerobic and aerobic cell-metabolism in cytoplasma respectively in mitochondriae means increasing the ATP-production of the cells. So more energy is available for all energy depending reactions. For instance more energy for synthetic reactions (protein biosynthesis), for catabolic reactions, energy for maintaining concentration-differences between the cell compartments etc.
The cell-metabolism-activation by H 2000 can also be demonstrated by growth experiments in laboratory-tests with guppy-fish or with tadpoles (Xenopus), or in practical animal nutrition experiments. The activity of H 2000 (S 2000, Y 2000) can be easily evaluated determining the fermention increasing factor decribed in V 5,2 and in XXII SPECIAL PART (20):
As an example of the many feed experiments carried out in agriculture with the additive H 2000 (used in amounts of 0.1 to 0.2 %), a few results from piglet breeding are shown in Tables 5a, 5b. The improvements in the daily feed intake were much more pronounced at 58 % and 42 % than in cases where commercial feed additives were used so that feed utilisation improved by 18.7 and 19,5 %, respectively.
Table 5a: Experiments with piglets aged 21 days, weight 5 to 12.5 kg
|
|
Controls (none) |
Commercial preparations A B |
Test preparation 2 ‰ |
|
|
No. of animals/group |
24 |
24 |
24 |
24 |
|
Starting weight (kg) |
4.93 |
5.14 |
5.12 |
5.06 |
|
Duration (days) |
20 |
20 |
20 |
20 |
|
Final weight (kg) |
10.2 |
11 |
11.9 |
12.9 |
|
Total weight gain (kg) |
5,23 |
5,84 |
6.78 |
7.45 |
|
Daily weight gain (g) |
262 |
292 |
339 |
373 |
|
Relative difference (%) |
100 |
112 |
130 |
142 |
|
Feed intake/animal/day (g) |
429 |
465 |
480 |
492 |
|
Feed used per kg gain (kg) |
1.64 |
1.59 |
1.42 |
1.32 |
|
Relative difference (%) |
100 |
97 |
86.3 |
80.5 |
|
Improvement: |
|
|
|
|
|
Daily gain (%) |
- |
11.7 |
29.5 |
42.4 |
|
Feed utilisation (%) |
- |
3 |
13.7 |
19.5 |
Table 5b: Piglets for breeding (10 to 35 kg)
|
|
Controls (none) |
Commercial preparations A B |
Test preparation 2 ‰ |
|
|
No. of animals/group |
24 |
24 |
24 |
24 |
|
Starting weight (kg) |
10.2 |
10.1 |
10.3 |
10.2 |
|
Duration (days) |
35 |
35 |
35 |
35 |
|
Final weight (kg) |
25.4 |
26.7 |
30.2 |
34.2 |
|
Weight gain (kg) |
15.2 |
16.6 |
19.9 |
24.0 |
|
Daily weight gain (g) |
434 |
474 |
569 |
686 |
|
Relative difference (%) |
100 |
109 |
131 |
158 |
|
Feed intake/animal/day (g) |
908 |
971 |
1.093 |
1.194 |
|
Feed used per kg gain (kg) |
2.09 |
2.05 |
1.92 |
1.74 |
|
Relative difference (%) |
100 |
98 |
92 |
83.3 |
|
Improvement: |
|
|
|
|
|
Daily gain (%) |
- |
9.21 |
30.9 |
57.9 |
|
Feed utilisation (%) |
- |
2 |
8 |
16.7 |
The commercial preparations were A: CTC and B: bayo-nox (also in Table 5a).
In V 6 fig 3-6 you find some TLCs of the new yeast cell preparations described in DE 4042157C 2,
The author has reported about his investigations concerning H 2000 –
properties and field results, e.g.in the
The patents, cited in (8), are protecting all kind of plants one can imagine. In the examples are descriptions of preparations from potatoes, soj germs, white cabbage, different yeasts. Further we tested six of the yeasts mentioned in VI 3 and seventeen other plants, mostly plants serving as human nutrition. The results will be published in a following paper “revealing a very general valid principle” which the author has been studying for many, many years.
IV 4
Basis: Cerealien (11a-d)
Vegetal placenta extracts, method for the production and use thereof
EP 1 663 274 from Aug 20th, 2003 (inventor: author), ms 63p + 3 p figures, 17 claims (11a) -
preceeding reports and mss in (28, 29).
Abstracts:
The invention relates to vegetal placenta extracts, particularly extracts from cereals, which are produced from the corresponding plant organs or suitable vegetal parts or from cell lines of the corresponding organs and/or vegetal parts, methods for the production and use thereof, either individually or in the form of combinations of pharmaceutical, cosmetic, dietetic and other compositions, especially in the form of capsules containing said products in an undiluted or diluted form, for oral administration.
A few examples of the extracts described in this patent specification (direct or via a cell culture):
Aqueous placenta extracts from rye, wheat, rice, triticale and rye-placenta extract - oil-soluble according to claims 1 to 8. Claims 9 to 17 relate to applications: Plate 6
Plate 6:
Claims of the patent EP 1 663 274 B1
1. A process for preparing placenta extracts of cereals, selected from the group consisting of wheat, rice, barley, rye, oats, sorghum (millet), triticale and spelt, from placenta cells of specifically prepared or commercially available placenta cell lines of the said cereals, characterized by the following steps:
a) activation of the selected placenta cell line of one of the cereals, selected from the group consisting of wheat, rice, barley, rye, oats, sorghum (millet), triticale and spelt, in a water bath at a temperature of preferably from 25 to 30 °C,
b) addition of a pre-_heated culture medium which is prescribed for the selected cell line by mixing it therewith and then incubating it in a manner known per se, preferably at a temperature of about 37 °C and preferably in an atmosphere containing about 5 % CO2,
c) separating and using the obtained cells as starting material for mass cultures in suspension by decanting the culture medium, washing the produced cell mass with a suitable buffer and introducing the cells into a complete medium and adjusting a dilution which is suitable for the mass production,
d) cultivating the cells in suspension culture in a large scale in a manner known per se by inoculating a cell
suspension in a sterile CO2/air mixture into a cultivating apparatus,wherein by introducing a plasmide and by DNA analysis it can be shown that the cells cultivated in large scale correspond to those of the initial cell culture;
e) preparation of an extract by treating the suspended collected cell material in an organic solvent and/or in
water, separating aqueous phase, preferably by centrifugating, removing residual lipid moieties (residue I) from the aqueous phase, optionally by multiple treatment with the organic solvent, for forming an aqueous raw extract, optionally in the form of a suspension,
f) purification or treatment of the raw extract depending on the starting material; and
g) sterile filtration of the extract, preferably by using a 0.2 Pm Millipore® filter or a PALL-_candle, after having adjusted the pH of the extract to a desired value of preferably 5.0 to 7,2.
2. The process according to claim 1, characterized in that a vegetal cell mass obtained in step (d) is suspended in ethyl ether or chloroform and stored for about 8 to about 15 days at a temperature of about -10 °C to about -20°C in a cooling room and then centrifugated for separating the aqueous phase.
3. The process according to claim 1 or 2, characterized in that the last traces of ether or chloroform are removed from the dialysate (outer solution) in step (f) by introducing nitrogen at pH 7.2 to 7.5.
4. The process according to at least one of claims 1 to 3, characterized in that as a cell line a placenta cell line of the pericarp (Fruchtschale), the testa (Samenschale) or the protein-_rich aleuron layer (Aleuronschicht) of cereals, selected from the group consisting of wheat, rice, barley, rye, oats, sorghum (millet), triticale and spelt, is used.
5. The process according to at least one of claims 1 to 4, characterized in that, if the cell wall material is important, the cell material suspended in water or in an organic solvent in step (e) is broken open in an ultrasonic generator and the obtained material after having been centrifugated and washed with water is treated by chemical and/or enzymatic partial hydrolysis and dialyzed.
6. The process according to at least one of claims 1 to 5, characterized in that for preparing vegetal placenta extracts from cell lines of cereals such starting materials are used which have not been subjected to a gene manipulation and which are free of undesired substances, especially free of immunogenic and/or pathogenic proteins (prions) and protein degradation products, and wherein no residues of insecticides can be detected.
7. The process according to at least one of claims 1 to 6, characterized in that one or more sterile filtrated vegetal extracts, which have been produced from one or more different vegetal cell lines, are prepared and optionally combined with one or more sterile filtrated animal organ extracts, which have been produced from one or more different animal cell lines, and optionally combined with one or more different, synthetically prepared animal organ extracts, and/or with one or more different animal organ extracts, which have been produced from fresh animal organs, and/or optionally with one or more vegetal extracts which have been produced from one or more different fresh organs or parts of plants.
8. The process according to claim 7, characterized in that the weight ratio between the vegetal placenta extract(s) and the animal organ extract(s) is (1 to 99) : (99 to 1), preferably (1 to 50) : (50 to 1), especially (1 to 20) : (99 to 80).
9. Use of the cereals placenta extracts and extract mixtures according to at least one of claims 1 to 8 for preparing a composition having a radical removing effect, in particular for cosmetic and/or technical purposes.
10. The use of the cereals placenta extracts and extract mixtures prepared according to at least one of claims 1 to 8 for preparing a cosmetic formulation.
11. The use according to claim 10 for preparing a cosmetic formulation having properties which inhibit the melamine formation (whitening effect).
12. The use of the cereals placenta extracts and extract mixtures prepared according to at least one of claims 1 to 8 for preparing a medical preparation for the topical, subcutaneous, intravenous or intramuscular administration for the healing of outer or inner wounds, for treatment of arteriosclerosis or of radiation damages.
13. The use of the cereals placenta extracts and extract mixtures prepared according to at least one of claims 1 to 8 for preparing a medical preparation for improving the immune system, for activating of the cell metabolism or for the treatment of gastroenterological diseases, in particular ulcera.
14. The use of the the cereals placenta extracts and extract mixtures prepared according to at least one of claims 1 to 8 for preparing a composition having an aphrodisiacatic effect.
15. The use of the the cereals placenta extracts and extract mixtures prepared according to at least one of claims 1 to 8 and of the raw extracts and precursors contained therein for stabilizing and improving durability of colored fabrics and color paints, especially lacquer works.
16. The use of the cereals placenta extracts and extract mixtures prepared according to at least one of claims 1 to 8 for preparing cultivation solutions, nutrition solutions and dietetic solutions.
17. The use according to claim 16 for preparing nutrition solutions and dietetic solutions in the form of capsules containing them in an undiluted or diluted form for oral administration, in particular as foodstuff complements.
To describe this patent and its applications we follow the lecture,
given by the author in May 1996 in a colloquium of the Chemical Central
Institute of UFSM Santa Maria, RS,
We developped grain-placenta preparations, gained from grain carpel brans, incl aleuron proteins, directly and/or via cell culture, under patent protection since 2003 (11a). Concerning rye-placenta cf illustrations 1a/b and Table 6.
The vegetal placenta extracts are able – like the animal ones (12-14) - to increase cell metabolic activity (cell stimulation). This has been proven for rye placenta extracts in WARBURG experiments (Table 7) and in growth tests as metamorphosis acceleration of tadpoles (Table 8).
Analogue to rye placenta preparations (Table 6) we prepared other cereal placenta extracts from bran layers (incl their aleuron cell layer) based on triticale, corn, oats, sorghum, spelt, rice, wheat, barley (11a-d).
Illustration 1:
1b Encyclopedia Britannica, Inc 1996
a) 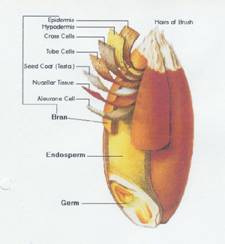
b) 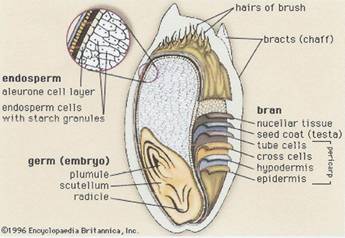
key to illustrations 1a and b next page.
Key to illustrations 1a and b:
The rye bran is the hard, browish outer protective skin of the grain; it surrounds the germ and the endosperm.
The bran consists of seven layers which are a concentrated source of dietary fiber; the layers with placenta character are collected together with the aleurone cells.
The inner part of the grain is the endosperm containing proteins and carbohydrates (from the endosperm the flour is produced).
The germ contains the plant embryo.
Properties incl. toxicological results for one of our prepared „Rye Placenta-Extracts“ are shown in Table 6:
Quality standard of „Rye-Placenta-Extract“ with dermatologic compatibility data for CELLRYEL (SERURYEL)
pH 6,75
dry substances 1,8 %
N content > 1,8 mg/ml
Identification:
WARBURG factor > 1,8 (liver homogenate = 1,0)
(respiration increasing factor)
amino acids ninhydrine positive (TLC)
peptides biuret positive
proteines protein-free
nucleic acid components positive (TLC)
Purity:
heavy metals < 20 ppm
arsen < 2 ppm
sterility germfree, prion-free
pyrogenes pyrogenfree
Toxicology:
acute toxicity (150 mice) DL50 i..v. : >25ml/kg
peroral : >25 g/kg
chronic toxicity (rats, dogs) no toxicity
reproduction, fertility,
teratogenity (rats) no impairment
teratogenity for rabbits (2,4kg) no negative findings (20 animals)
The dermatologic compatibility have been tested in the usual way. The Rye Placenta Extract (Table 6) passed all tests without showing any irriation and without any negative results:
- Open epicutaneous test of sensibilisation of guinea pig for 3 weeks according to BÜHLER,
- eye irritation test with rabbits according to DRAIZE,
- examination of primary skin irritation with rabbits (DRAIZE),
- clinical-allergological examination: PATCH-test with human beings.
In addition: AMES test for mutagenic actitivity, carried out without and with metabolic activiation (screening).
Quality standard of “Triticale-Placenta-Extract” (TRICELL) in (11d).
The methods for examination of the metabolism activating properties of Rye Placenta Extract are:
1) Measurement of the increase of tissue respiration, e.g. of a liver homogenate of a rat in the WARBURG-test for determination of the respiration increase factor: the respiration of the liver homogenate is set to be 1,0 (control)
2) Growth tests: Tadpoles of Xenopus laevis DAUDIN by measuring their length, the increase of their weight and the advances of the metamorphosis to a frog (start and end of the conversion to a frog) in days
Results to 1):
The factor for increasing respiration of the Rye Placenta Extract RP 17 equals 1,83: Table 7
Table 7: Record of the WARBURG-factor determination
Result: factor for rye placenta extract: 1,83 – control set to 1,0
(pre-incubation time 1 hour at 37°C)
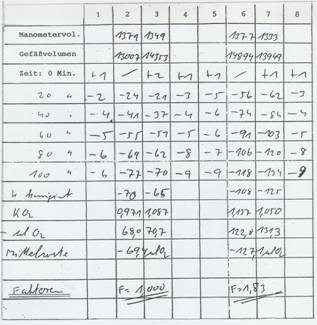
Method described in the patent EP 1 663 274 B 1 (11a); cf. also method description in (13).
Results to 2):
Table 8: Influence of the „Rye-Placenta-Extract” CELLRYEL on the metamorphosis acceleration of tadpoles Xenopus laevis DAUDIN
Experimental group 1 2 3 4
d 49 1+0 1+0
d 53 0+1 1+0 1+0
d 56 0+2 0+1 1+1 1+1
d 58 0+3 0+1 1+1 1+2
d 60 0+3 0+1 1+2 1+2
d 63 0+3 1+1 2+3 2+4
d 64 1+3 1+1 2+4 2+4
d 67 2+2 1+0 2+6 3+5
Column 1 and 2 : controls
column 3 and 4 : with “Rye-Placenta-Extract” RP 17
number pairs:
first number : number of frogs
second number : number of the metamorphosis (the metamorphosis is finished when the tail has been resorbed completely or almost completely)
Method described in the patent application under PCT number WO 2005/027946 A 1, pp 41-43, entitled “Vegetal-Placenta-Extracts. Method for the Production and use thereof” (11a).
Final remark:
In the mean-time the rye-placenta-based products are in trade in
The Rye-Placenta-Extract is an alternative to foetal calf serum extract CELLRYL (14)
In (9) reference has been made to additional plant extracts that can replace organ extracts: Colla 120 and COLLAPLANT PO (dealt with below); re Colla 120 from Sycamore cell culture see table VI in (2a).
IV 5
Cell wall glycoproteines – Collaplant PO (9)
COLLAPLANT PO is prepared from the starting material Platanus occidentalis, splitting the cell wall glycoproteins, which are rich in hydroxyproline and other collagen-amino acids, followed by hydrolysis, and deproteinization of the rest by citric cycle acids.Qualitätsstandard in Tab 9.
The preparation contains more than 100 components from the classes: carbohydrates, peptides, amino acids, nucleic acid components, acids, vitamins, minerals and trace elements. The peptides are particularly significant.
COLLAPLANT PO is a suitable substitute for animal collagen – regulating and ameliorating the humidity-maintaining capacity of the skin: Diag 3a.
Diag. 3a: Skin moisture measurements (in scale divisions of Corneometer M 420) of 20 probands (aged 40-70 years) with 1% and 3% COLLAPLANT PO emulsion and placebo up to 120 mins
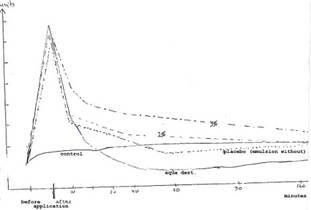
Key to Diag. 3a: The initial skin value (control) is almost constant. Aqua dest. shows clear “drying out” after approx 30 mins. The emulsion without COLLAPLANT PO gives 40-60 minute values that fall slightly below the initial skin values in the “drying out zone”. The moisture-retaining effect lasts over two hours with the 1% water/oil emulsion. With the 3% emulsion a clear increase of the initial skin value is discernible even after 120 mins.
Table 9a: Examples for quality standard of COLLAPLANT PO (9)
|
pH |
6,2 |
|
dry substances (%) |
6,3 |
|
N (%) |
0,42 |
|
amino acids (ninhydrin) |
+ |
|
peptides (biuret) |
+ |
|
hydroxyproline (%) |
>0,1 |
|
nucleic acid components |
+ |
|
melanoma B16 test (mouse) |
+++ |
|
Purity |
|
|
heavy metals (ppm) |
< 20 |
|
As (ppm) |
< 2 |
|
proteins |
protein-free |
|
Sterility |
germ-free |
|
Prions |
Prion-free |
Table 9b:
Whitening effect of COLLAPLANT PO, lot CP-1,
mentioned in Table 9a; detection method: melanoma B16-test. In the patent (19) relating the “whitening effect” of cosmetic additives you find a detailed prescription of the analytic method used to control melanin synthesis[4]; cf. APPENDIX VI 1.
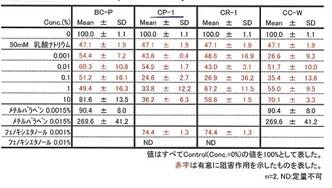
Diagram 3b:
COLLAPLANT PO accelerating the collagen synthesis in human fibroblasts
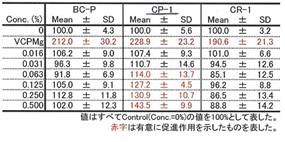
COLLAPLANT PO, lot CP-1 has a significant effect of collagen synhesis in fibroblasts: Diagram 3b and Table 9c.
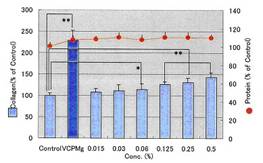
Table 9c:
Experimental data to diagram 3b
Many animal extracts have the "whitening effect" desired by the cosmetic industry. Again, our search for alternatives led us to a synthetic solution as shown in APPENDIX VI 1; ref (19).
V SPECIAL PART:
V 1: Determination of the respiration-enhancing factor
V 2: Determination of the amino acid oxidase-inhibiting factor
V 3: Measurement of wound healing activity
V 4: Influence on the reticuloendothelial system (RES)
V 5,1-5,4 Excerpts
from the
V 6,1 TLCs: Fig 3-6
V 6,2 Cytocatalyzer
V 1
Determination of the respiration-enhancing factor
Experimental details on (A) see section IV 2, following right after the Abstract
The respiration-enhancing factor for yeast preparations with metabolic activity is determined on the basis of the effect on rat liver homogenate according to the WARBURG method and shows the ratio of the oxygen additionally consumed by the standardised rat liver homogenate vis-à-vis a control of the same composition, but not including such a preparation.
The measurement of the enhancement in respiration and of the respiration-enhancing factor is carried out in a WARBURG vessel of a certain volume filled with a buffer (preferably Sörensen buffer of pH 7.4), a specific amount of conditioned rat liver homogenate and the test substance or test solution in accordance with a feed plan. The test substance usually acts on the homogenate at 37°C for a period of 60 minutes. The O2 consumption is determined by a manometer reading after the CO2 resulting from respiration has been adsorbed quantitatively. The oxygen consumption converted to normal conditions, divided by the consumption of a control sample without the test substance, is a direct measure for the respiration-enhancing factor.
V 2
Determination of the amino acid oxidase-inhibiting factor
Experimental details on (B) see section IV 2, following right after the Abstract
In order to determine the L-amino acid oxidase-inhibiting factor (Hmf) of the yeasts of the invention, L-amino acid oxidase solution (1 mg/ml), catalase suspension, buffer pH 7,8 (50 ml 0.2 M Na2P2O7 + 37.5 ml 0.2 M HCl), L-phenyl alanine solution (1 mmol/50 ml, buffer 7.8) and test solution (centrifugate, 103 g, or a yeast suspension) are placed into a WARBURG vessel in accordance with a specific feed plan so that the measurements record both the reaction of L-amino acid oxidase and L-phenyl alanine and the reaction of the enzyme with the test solution (without phenyl alanine) and of the enzyme with the test solution and phenyl alanine. The cylinder inserts are equipped with strips of filter paper saturated with 6 N KOH. The solutions are equilibrated at 37°C; then the enzyme solution is added to the main space containing the test solution or a blind solution to start the reaction. The consumption of oxygen in µl may be recorded as dependent on time and is a direct measure of the inhibiting factor when placed in correlation with the oxygen consumption of the blind experiments. In general, the reading used to determine the Hmf factor is carried out after 60 min.
For evaluation, the measurement graphs "L-amino acid oxidase + phenyl alanine" and "L-amino acid oxidase + yeast solution" are added up to form an overall graph - the oxygen requirement is due to the amino acids contained in the yeast itself - and then places the overall graph which is a measure for the oxygen uptake expected theoretically in correlation to the graph "L-amino acid oxidase + L-phenyl alanine + yeast solution". In case of the yeasts of the invention (subjected to biophysical derivatisation), a reduced consumption of oxygen results which expresses the inhibiting factor in percent (e.g. 0.42 = 42 % inhibition or reduced oxygen consumption). Compared with the respective starting yeasts, which practically correspond to the theoretical overall graphs, the Hmf is significant and achieves values of up to 70 or even 80 %.
In APPENDIX VI 2 an example is described in detail.
V 3
Measurement of wound healing activity
Experimental details on (C) see section IV 2, following right after the Abstract
Another surprising characteristic of most of the yeasts of the invention is their wound healing activity which may be evaluated on the healing of cuts on the back of rats after 6 days. The tension at the wound site of the animals treated was significantly increased over a control group. Remarkably, he superiority of the animals treated vis-à-vis the control group was still measurable in a test on day 21.
In this test, male Wistar rats weighing 210 to 230 g were anaesthetised and the hair on their backs was shorn. Then a straight incision of approx. 4 cm in length was made along the medial line and sutured in three places spaced apart by approx. 1 cm. On days 6, 11, 16 and 21 after the procedure, the wound was opened and 3 narrow slivers of skin taken at a right angle to the wound line including the 1 cm width of the wound line. Then the tension resulting from peeling off the used portion was measured.
2.5 mg/kg of the centrifuge supernatant of the test solution from example 1 were administered on the day of the incision and then daily afterwards. The control group received an application of 2.5 ml/kg of physiological saline in an identical manner. 25 animals per group were tested during each examination.
It was possible to observe that the tension of the wounded skin area increased uniformly in the animals treated with the test solution. During each daily examination, the animals treated showed higher tension of the skin area than the control group. During the early stage on day 6, the difference to the control group was more than 100 %. The results are summarised in Table 10:
|
Number of days |
Test group*) |
Control group |
|
6 |
215 ± 17 |
104 ± 33 |
|
11 |
493 ± 24 |
390 ± 23 |
|
16 |
840 ± 48 |
730 ± 30 |
|
21 |
1.659 ± 52 |
1.401 ± 60 |
*) The test group received 2.5 ml of test solution/kg weight.
V 4
Influence on the reticuloendothelial system (RES)
Experimental details on (D) see section IV 2, following right after the Abstract
Influence on the reticuloendothelial system. Owing to their effect on the RES function in rats inhibited by Benzamine Blue (Tryphanblau) according to the Congo Red method (Kongorot-Methode), an RES-activating activity can be determined for the new yeast preparations. For this purpose, male Wistar rates weighing 210 to 230 g were injected intravenously with 1 ml/kg of 1% Congo Red solution; blood samples were taken after 4 and after 60 minutes. The Congo Red coefficient resulted from the ratio of the decadic absorbance at 500 nm of the serum diluted tenfold. In order to inhibit the function of the RES system, 10 mg/kg of 1% Benzamine Blue solution were administered by the intraperitoneal route. By measuring the decadic absorbance at 650 nm, it was possible to calculate the inhibition as a corrected decadic absorbance at 500 nm. The Benzamine Blue administration took place 6.5 hours before examining the RES system with Congo Red. In this test, 0.5 ml of the test solution (centrifuge supernatant according to example 1) were administered intraperitoneally 5 hours before the examination. The control group received an adequate amount of physiological saline. 25 animals were tested per group.
We found a mean Congo Red coefficient of 9.4 for the group treated, and a mean Congo Red coefficient of 16.1 for the control group which can be regarded as direct proof for the increasing activity of the yeasts according to the invention on the function of the RES system, because the inhibiting effect caused by Benzamine Blue is cancelled.
The increased metabolic activities shown for the yeast preparations of the invention are totally surprising insofar as the yeasts and yeast preparations are exclusively treated by physical methods. This treatment is generally called "derivatisation". The cells may be derivatised by the influence of cold, laser radiation, sonification and other biophysical methods as well as by a comparatively short-term intense heat treatment. It seem that this causes a significant reduction in size of the yeast cells, preferably to the 0.5 fold or less, while the cell walls become stronger so that the ratio of the mean cell diameter/cell wall thickness is changed by a factor preferably ranging between 5 and 20.
V 5:
V 5.1: Example 9 from the PR-China-Patent: Plates 7a and 7b
V 5.2: Increase in fermentation by yeast preparations according to the patent application (8a,b)
V 5.3: Claims of the PR-China-Patent: Plate 8a and 8b
V 5.4: ATP formation
V 5.1: Example 9 from the PR-China-Patent 91105974.1: Plates 7a and 7b
In the patent from the
Plate 7a:

Plate 7b: Example 9 (German translation of Chinese text) [documents]

V 5.2: Increase in fermentation by yeast preparations according
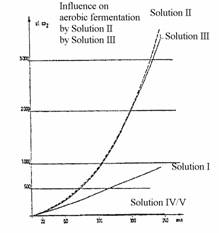
to the patent application (8a)
Diagram 4: Determination of the fermentation increase factor
(Translation in Diagram 1)
The fermentation increase factor is the ratio of the CO2 volume released from a fermentation system in a WARBURG experiment based on the CO2 volume generated by the same system without addition of a preparation. The comparative basis is therefore the model of a conventional fermentation.
The measurement of the fermentation increase factor is carried out in a WARBURG vessel of a specific volume charged with a solution consisting of 2.5 g of glucose, 0.4 g of casein peptone, 50 mg of MgSO4, 7H2O and 50 mg of baker's yeast ad 50 ml of tap water. The vessel is then charged with a specific amount of the test preparation according to a feed schedule. The amount of CO2 released at 30°C is determined depending on the time, converted to normal conditions and divided by the standardised CO2 amount generated in the control experiments under the same conditions, but without a test preparation.
The measurable fermentation activity is remarkable for the preparations of the invention as shown by the following Table 11:
|
Sample No. (origin) |
Fermentation increase factor |
|
Control |
1.0 |
|
Preparation (Example 1) |
3.83 |
|
Preparation (Example 2) |
3.61 |
Diag. 4 of the drawing shows the development of the CO2 formation in the WARBURG experiment depending on the time. Two groups of comparative experiments are used, i.e. the test solution without the preparation which characterises the progress of conventional fermentation and the yeast-free test solution with the preparation which does not result in any significant influence on fermentation.
The solutions were composed as follows:
Solution I: 50 ml of basic medium + 50 mg of baker's yeast
Solution II: 10 ml of solution I + 50 µl of the preparation of Example 1
Solution III: 10 ml of solution I + 50 µl of the preparation of Example 2
Solution IV: 10 ml of basic medium + 50 µl of the preparation of Example 1
Solution V: 10 ml of basic medium + 50 µl of the preparation of Example 2
The basic medium solution has the above-mentioned composition used for the standard experiments to determine the fermentation increase factor.
[Part II of this two-part paper will be featured in the upcoming September-October issue of this Journal.]
REFERENCES - by the author (co-workers in brackets)
(1) Wässrige synthetische Organextrakte,
EP 0 552 516 B1 from 02.12.1992 (inventor: the author), 38 p
published: 28.07.1993, Patentblatt 1993/30, 25 claims, amed states: AT,BE,CH,DE,DK,ES,FR,GB,IT,NL,SE
(2a) Wasserlösliche Organextrakte mit verbessertem biochemischen Wirkungsgrad, Verfahren zu ihrer Herstellung und ihre Verwendung,
DE 196 24 476 C2 from 19.06.1996 (inventor: the author), 54 p, published: 02.01.1998, 33 claims
CH 692 408 A5 – published: 14.06.2002
In these patents the author gave a very detailed description of all experiments belonging to the processes and uses of the 33 claims including eleven examples.
(2b) Cell line based organ material beats BSE risk in animal organ extracts,
Relata Technica Web Site http://www.vevy.com/relata, issues 2001, 3 p
International Electronic Journal on Dermopharmacological Research, Dermopharmaceutical Technology and related Subjects
(3) Manufacturing method for PORCINE-Placenta-Extracts,
Brasilian Patent Application P 1 601 34 02 from 15.06.1961 [co-workers: F.R. Pesserl, M.M. Faria]
(inventor: the author), 12 p;
produced from 2001 on in
(4a) Verfahren zur Herstellung eines stabilisierten wasserlöslichen Plazenta-Extraktes,
DE 2 338 970 C 2 vom 01.08.1973 (inventor: the author), 8 p
Verfahren zur Herstellung eiweissfreier Präparate,
DE 2 021 969 C 2 vom 29.04.1970 (inventor: the author)
(4b) Porcine based organ Extracts guarantee full substitution of Bovine extracts,
Relata Technica Web Site http://www.vevy.com/relata , issues, 2001, 2p
International Electronic Journal on Dermopharmacological Research, Dermopharmaceutical Technology and related Subjects
(5a) Verfahren zur Herstellung thallophytischer oder bryophytischer Zytorrhysate, danach hergestellte Zytorrhysate und deren Verwendung zur Nährstoffergänzung
EP 0 208 805 A1 vom 19.07.1985 (inventor: author) 34 p, 21 claims
(5b) Verfahren zur Herstellung thallophytischer oder byrophytischer Zytorrhysate, danach hergestellte Zytorrhysate und deren Verwendung zur Nährstoffergänzung = Chemizytorrhysate,
DE 3 402 169.8 vom 23.01.1984 (inventor :the author), 48 p
published: 08.08.1985: DE 3 402 269 A 1.
13claims
Japanisches Patent 9807/85 from 22.01.1985 (inventor: author)
(5c) Teilung aus DE 3 402 169.8 :
Aphrosisiakum zur Steigerung der Potenz: DE P 3 448 223.7 C2 vom 23.01.1984 (inventor: author), 5 p, 1 claim, 3 examples,
Zytorrhysate zur Nährstoffergänzung zum Giesswasser: Pflanzenwuchsstoffe aus thallophytischen und bryophytischen Cytorrhysaten; [12 photographs in (18)]
DE P 3 448 283.0 from 27.08.1988 (inventor: author)
(6) “Placenta-Extracts without alkaline phosphatase” (experimental data 1976-86), lecture held by the author in Portugese on Sept, 1986 in UFSM, Instituto Central de Quimica, S. Maria, RS, Brazil; ms 15p + 6 tables
(7a) Biophysikalisch derivatisiertes Hefepräparat. Verfahren zu dessen Herstellung und dessen Verwendung als Futtermittelzusatz,
DE 3 711 054 C 2 from 02.04.1987 (inventor: author), 13 claims
Applications in other countries, i.e.:UdSSR v/o patent 43550 14/13 (000127) from 14.01.1988
Poland P 271 547 from 31.03.1988, Hungary P 2829/88, CSSR PV 2234/88, Denmark S 1936 AU, from 21.09.1989, Australia 15771/88 corresponding PCT/DE 88/00212 from 31.03.1988, Portugal P 37110543 from 02.04.1987
(7b) Biophysikalisch derivatisiertes Ascomycetes-,. Schizomycetes- und Hefe-Präparat enthaltende Futtermittel und Pflanzenwuchsstoffe, sowie Verwendung der Präparate zur Hautbehandlung und probiotischen Aktivierung
EP 0 286 033 A2 from 31.03.1988 (inventor: author), 13p, 30 claims, 1 fig., 6 examples
(7c) Leveduras biofisicamente transformadas,
Brasilian Patent application P 1 860 3405 from 31.03.1988 (inventor: author), 15 claims
(8a) Gärungssteigernde Zellpräparationen auf Pflanzenzellbasis
DE 4 042 157 C 2 from 28.12.1990 (inventor: author), 14p, 14 claims, 8 fig
(8b) Gärungssteigernde Zellpräparationen auf Pflanzenbasis
Swiss Patent 684 274 from 24.12.1990 (inventor: author), 18 p, 30 claims, 8 fig
(8c) Chinese Patent (VR) 91 10 59 74.1 from 23.08.1991 (inventor: the author), 29 p, 30 claims, 8 figures
There is a special
report entitled “Fermentation increasing cell preparation on vegetable basis-process
Y 2000” to evaluate the
(8d) A plant cell preparation and its manufacturing method,
Japanese Patent 90058/92 from19.03.1992 (inventor: author)
7 claims
(9) Cytocatalyzer, Cellryel, Collaplant PO – plant-based cosmetic additives
http://www.bwwsociety.org/journal/html/cytocatalyzer.htm 2003, 6p
The BWW Society/The Institute for Advancement of Positive Global Solutions
(10) Two Notes on Progress in BSE-Crisis
http://www.bwwsociety.org/journal/html/bsicrisis.htm 2002
The BWW Society/The Institute for Advancement of Positive Global Solutions
(11a) Vegetal Placenta Extracts, Method for the Production and the use thereof,
EP 1 663 274 B1 in DE and in CH from 20 Aug 2003, (inventor: the author) ms 63p + 3 p figures
17 claims, Plate 6
International number WO 2005/027946 B 1, 31.03. 2005 Gazette 2005/13
In the descripton of the patent you find some preceeding older references which do not touch the invention though.
(11b) «Vegetal Placenta Extracts»
Lecture, given by the author in May 1996 in
the colloquium of the Chemical Central Institute of Universidade Federal de
(11c) Vegetal Placenta extracts substitute animal Placenta extracts
http://www.vevy.com/relata/issues.articles 2008, 6p
International Electronic Journal on Dermopharmacological Research, Dermopharmaceutical Technology and Related Cosmetic Subjects
(11d) Cytocatalyzer, Cellryel, Tricell, additivi cosmetici a base vegetale
homepage: www.riemschneider.org 2001; cf. also (28,29)
(12) Metabolic-aktivating organ extracts
Institute for Biochemistry, FU Berlin and Chemical Central Institute, UFSM [co-worker: Th. Wons]
Cosmetics and Toiletries 94, November 1979, pp. 71 – 76.
(13) Über Methoden zum Nachweis einer erhöhten Aktivität von Stoffwechselvorgängen unter dem Einfluss von Organextrakten
RAK (Riechstoffe, Aromen, Kosmetik) 1977,236-240 and 246-250 [co-worker: K Hennig];
cf DE patent 2405983 from 8 April 1974 (inventor: Riemschneider)
(14) Production of a standardizable proteinfree extract from hemolyzed blood and foetal calf serum), Japanese Patent 94266/73 from 22 Aug 1973 (inventor: Riemschneider)
cf also: Monography entitled “Ulcustherapeuticum CELLRYL” 1977, Teikokuzoki Pharm.K.K., Tokyo, with 19 clinical original papers;
cf also PROJ XXIII in „Re-reading - 66 years chemistry“ [in preparation (20)]
(15) Bonded to people – ADDENDUM
http://www.bwwsociety.org/journal/html/bondedtopeople.htm 2008/9 - ADDENDUM in Part III
The BWW Society/The Institute for Advancement of Positive Global Solutions
(16) Useful and questionable applications of several vitamins and anti-vitamins
http://www.bwwsociety.org/journal/html/vitamins.htm 2008
The BWW Society/The Institute for Advancement of Positive Global Solutions
(17) Nutritional Supplement by Hydrid Ions acting as Antioxidants and Hydrid Ions and H Atoms as "Energy Currency" for Living Systems
http://www.bwwsociety.org/journal/html/hydrid.htm 2004
The BWW Society/The Institute for Advancement of Positive Global Solutions
(18) PLANT TECHNOLOGY based on Chemistry, Botany, and Architecture
http://www.bwwsociety.org/journal/html/planttech.htm 2008
The BWW Society/The Institute for Advancement of Positive Global Solutions
(19) Kosmetische Zusammensetzung mit Whitening-Effekt. Verfahren zu ihrer Herstellung und ihre Verwendung
EP 03 718 674.9 - PCT/EP 03/01874 from 24.02.2003 (inventor: the author) 28 p, 15 claims
In memoriam:
This article is dedicated to the author’s long-standing Brazilian friends M.M. Faria and J.M. Faria, who unfortunately died in the crash of a private plane belonging to colleagues.
[1] Adress correspondence to Prof. Dr. Dr. R. Riemschschneider, D-14001 Berlin, Postfach 1164, Germany
[2] BSE = bovine spongiforme encephalopathie (10)
[3] Definition of “ortho-molecular medicine” according to R.Rowghani in (15): Regarding human health, L. Pauling is working on the assumption that „we find well ordered molecules in all cells of the body, a state to be achieved which Pauling called ORTHO-MOLECULAR in the sixties (όρϑός = right; molecular = regarding molecules). This is the origin of ORTHO-MOLECULAR MEDICINE which has become well known in the meantime and is applied by doctors. It says that an individual will fall ill if he does not have all the necessary molecules in the cells of his body, which results in malfunctions first and often in serious diseases afterwards“.; cf. ADDENDUM in (15).
Aqueous synthetic proteinfree organ extracts (1) opened the possibility to „smuggle“ lacking important molecules into the organisms.
[4] Pictures relating to the evaluation of the melanin synthesis in B16 melanoma cells have been omitted.
[ BWW Society Home Page ]
© 2009 The Bibliotheque: World Wide Society