Commentary: Autobiography:
Bonded to People, Part III
Central Institute of Chemistry, Universidade Federal de Santa Maria (UFSM), Santa Maria, Rio Grande do Sul, Brazil
BWW Life Fellow Member Professor Randolph
Riemschneider has summarized his lifetime work in a soon to be published book
[1] in which readers will find some enlightening reports about the numerous
people who influenced his life. With this paper the author also continues his
essay “Bonded to Compounds –
Chemistry has always been and remains the author’s life. For the sake of his devotion to chemistry, the author has resisted and confronted all problems which politics can involve, and has fought against resistances and intrigues. Here it is shown from whom and in what manner he received support and sorely-needed assistance.
This essay also represents a piece
of contemporary history, especially from the 1940s to the 1960s, regarding
Nobel Prize winners such as Profs Alder/Diels, Butenandt, R.Kuhn, Pauling,
Natta/Ziegler and in Germany well-known personages such as Profs. Dinghas
(mathematician), Lohmann (ATP), Sauerbruch, General Nobile, Dir. Dr. Scherer
(Freon HOECHST), and Dr. Ronge (attorney) as well as personages from
In Norway, a hexachlorocyclohexane having a melting point of 146°C (I) was isolated from cyclohexane chlorination products and was deemed to be another stereoisomer of the C6H6Cl6-isomer of the 1,2,3,4,5,6 series resulting from the chlorination of benzene. O. Hassel determined its dipole moment, i.e. of I, and placed I by mistake as a zeta-isomer in the series of the five stereoisomers alpha, beta, gamma, delta, epsilon already known without checking by a simple reaction which positional isomer it actually was.
[For reference:
E) eta-1,2,3,4,5,6-Hexachloro-cyclohexane (eta-I), chair-configuration 1e2e3e4a5e6a (and zeta-I: 1e2e3a4e5a6a)
Constitution of eta-I:
That the new isomer eta-I belongs to the 1,2,3,4,5,6-serie is shown by the treatment with Zn-dust (→ benzene), and with alkali (→trichlorobenzenes), carried out with micro amounts
Configuration of eta-I:
Determined by dipolmoment measurements: e.e.e.a.e.a, found: 3,2 D, calc. 3,5 D [14b]. RAMAN-spectra - 611b in [1].
With exception of the isomer e.a.e.a.e.a all theorectical possible chair-configurations of the 1,2,3,4,5,6-series were isolated and determined; so performed from the author on 15 Aug 1964 in the colloquium held in Sala de Atos, Edificio sede da UFSM, Santa Maria, RS, Brasil
List of the isolated C6H6Cl6-isomers
alpha - C6H6Cl6 mp. 159°C (e.e.e.e.a.a)
beta - C6H6Cl6 mp. 309°C (e.e.e.e.e.e)
gamma - C6H6Cl6 mp. 113°C (e.e.e.a.a.a)
delta - C6H6Cl6 mp. 139°C (e.e.e.e.e.a)
epsilon - C6H6Cl6 mp. 218°C (e.e.a.e.e.a)
zeta - C6H6Cl6 mp. 88/89°C (e.e.a.e.a.a) *
eta - C6H6Cl6 mp. from 70°C (e.e.e.a.e.a)
In Table 2a there will be shown the spatial structure of the seven isomers.
* zeta-I: The real** zeta-1,2,3,4,5,6-Hexachloro-cyclohexan, mp 88-89°C (zeta-I) is described in ref (608) in PROJ IX (1), here ref (20)
Our proof of constitution: zeta-I belongs to the 1,2,3,4,5,6 series because it is converted to benzene through the zinc dust treatment, cleaving 6 Cl atoms. When exposed to alkali it reacts, forming trichlorobenzenes (cleaving 3 HCl).
Our proof of configuration: Short-term chlorination of zeta-I in an open vessel to CCl4 resulted in an oil from which were obtained by means of chromatographic adsorption: delta-1,1,2,3,4,5,6-heptachloro-cyclohexane with a melting point of 138 to 140° C (delta-IV of the configuration ea.e.e.a.a.e) and alpha-1,1,2,3,4,4,5,6-octachloro-cyclohexane with a melting point of 93°C (alpha-III of the configuration ea.e.e.ea.e.a).
stepwise chlorination of zeta-I: zeta-C6H6Cl6 mp 88 - 89°C (e.e.a.e.a.a) to delta-C6H5Cl7 mp 138 - 140°C (ea.e.e.a.a.e) [delta-IV] to alpha-C6H4Cl8 mp 93°C (ea.e.e.ea.e.a) [alpha-III]
** About the so-called zeta-isomer, an C6H6Cl6 isomer[i] with Cl-atoms in the position 1,1,2,4,4,5 [and not in 1,2,3,4,5,6 as believed Hassel and co-worker and called it by mistake zeta-isomer] see 5 publications in [21] and also next page.
"zeta-Gezeter" (Gezeter: Much ado about nothing) over the zeta-isomer or the "zeta-isomers" of C6H6Cl6 (zeta-C6H6Cl6)]
A determination of the constitution always ranks before determination of the configuration (constellation)!
The author was able to put right this mistake when he early started systematic studies of the chlorination of cyclohexane and developed the so-called "cyclohexane family tree" of the chlorination products of cyclohexane: Table 2b. The so-called zeta-isomer of Hassel turned out to be 1,1,2,4,4,5-hexachloro-cyclohexane. The author proposed to eliminate the designation zeta for it and to use the letter zeta for the sixth stereoisomer of the 1,2,3,4,5,6 series by then actually isolated from the so-called delta-oils from the chlorination of benzene: zeta-1,2,3,4,5,6-hexachloro-cyclohexane having a melting point of 88 - 89°C and the chair configuration 1e2e3a4e5a6a [20].
Unfortunately, American experimentators caused some confusion by calling the sixth stereoisomer they isolated eta-isomer, even though they were aware of our experimental results. The author has given a comprehensive report of this case in PROJ IX in "Re-reading - 66 years of chemistry" and also described the real eta-isomer he isolated; cf. [20] and [21].
Table 2a: The isolated 1,2,3,4,5,6-hexachloro-cyclohexanes [16,15]
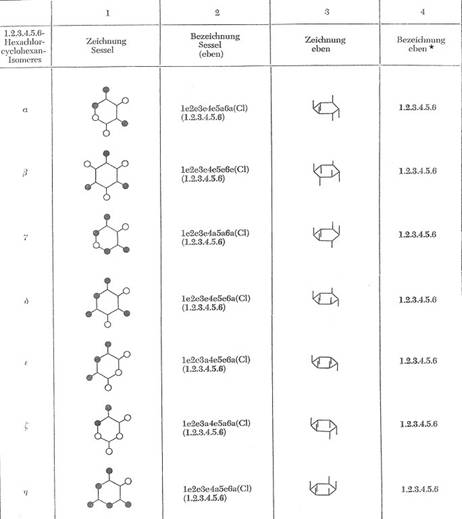
It is still lacking the eighth theoretically possible C6H6Cl6-isomer of the chair-configuration 1e2a3e4a5e6a. In case of realisation, it would be the theta-isomer.
Of the 1,2-dibromo-3,4,5,6-hexachloro-cyclohexanes we isolated 4 isomers: PROJ IX in [1].
Table 2b:
Cyclohexane chlorination products: "Cyclohexane family tree”*
The products obtained in crystallised form from the chlorination of cyclohexane are preferably Cl-substituted in the positions 1,2,4,5, i.e. some CH2 groups remain intact in favour of the formation of CCl2 groups: Substitution products bearing Cl atoms on all C atoms were not obtained until the Cl number 8 was reached: Plate 4:
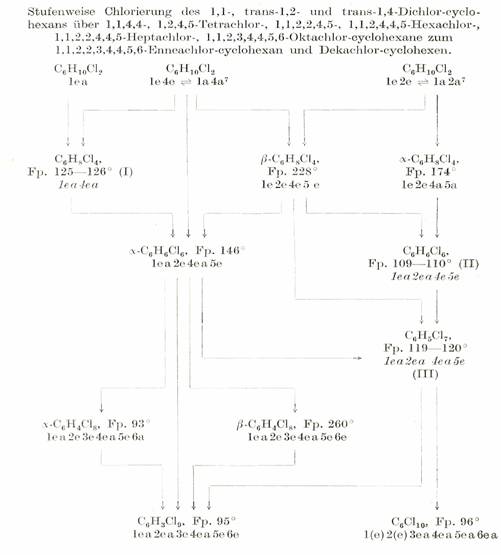
* Based on the results described in Mitt. XI, XII, XVI, XXXI, XXXII, XXXIII, XLVII, XLVIII, LI in [1]; also see summary in Mh.Chem.86, 101-116 (1955).
F) “Reactions in compressed CO2” [lecture 1] - “Inorganic-organic solvents having a low melting point (polar ionic, non-aqueous solvents)” [lecture 2]
When looking 1947 for suitable solvents for aminoacids +
hexachlorocyclohexane isomers, “low melting salts like alkylated imidazoles and AlCl3” turned to be useful: Inorganic-organic solvents: PROJ IX.
1949 we used compressed CO2 as a solvent and as a reaction medium, for instance : “C6H6Cl6-isomers + amino acids in CO2 with and without solvent addition” [10a] and other reactions in or with CO2; continued for many years (unpublished).
1998/99 we studied the reaction of compressed CO2 with several gas-clathrate compounds according to the following equation:
CO2 + Y(H20)x → Y + CO2(H20)x ,
in the case of Y = CH4 with the aim to gain CH4 from CH4(H20)x and simultaneously to eliminate CO2 - which would concile two things.
In our methan-ice-experiments we tried to find out the best pressure and temperature for the methane-reaction in presence and absence of NaCl [10b].
The method to use CO2 injections (CO2 as an "expensive" waste product) to improve mineral oil recovery[ii] has been in use since the nineteen nineties; for example, STATOIL-HYDRO has been depositing CO2 below ground in the Sleipnir West Field since 1996. Oil companies take the unpopular "anti-climate" gas and pump it into their reservoirs to improve oil recovery.
Those industries using methane ice will become a large CO2 consumer and thus CO2 customer.
Already at the beginning of our solvent experiments we made a big difference between “low melting ionic liquids (I , low viscous) and salt melts with high melting point (II, high viscous), when it was a question of the substitution of conventional organic solvents. Compressed CO2 (III) belongs to nonpolar solvents, I-systems melting below 10°C are to take into consideration as substitute for polar organic solvents.
The two lectures mentioned in the title were held at HOECHST on Dec 15, 1952 [10a]: Introduction and summary as memorandum
Content:
Starting in PROJ. IX with experiments about the reaction of the 1,2,3,4,5,6-hexachloro-cyclohexane isomers with elementary sulfur in melted form, the focus being on the search for a use and/or as a solvent of the alpha-isomer produced in large quantities as a "by-product". Attempts at reacting more than 240 compounds with the alpha- and gamma-isomers, including anthranilic acid and alpha-D and alpha-L-amino acids, but also beta-alanine and many peptides. Test protocols from the years 1948 to 1951, 41 pages (secreted). The reaction of alpha-isomer with sulfur produced very interesting results (secreted).
When gammexane is ground with anthranilic acid in ether a clear solution is produced which, after evaporation of the ether, yields a light-green product having a melting point of 126° to 127°C and containing 2 moles of anthranilic acid per 1 mole of gammexane. The bis-(anthranilic acid)-gammexane (I) obtained (molecule aggregate) dissolves in methanol/H2O, namely in 1 g I + 2 ml H2O + 5 ml of methanol. Dissolution stops when the water content exceeds 50 %. When looking for suitable solvents for alpha-amino acids + hexachlorocyclohexane isomers, "low melting salts" of alkylated imidazoles and aluminium chloride turned to be useful: "Inorganic-organic solvents"
In the lab report from the year 1949 prepared together with cand. chem. Hein, first tests regarding the use of compressed CO2 as a solvent: a) C6H6Cl6-isomers + amino acids in CO2 with and without the addition of benzene, other hydrocarbons and alcohols. b) 12 other reactions in or with compressed CO2 (secreted)
Manuscript (memorandum) 1950, 15 pages, based on the lectures held at Hoechst on December 15, 1952.
This memorandum resulting from experimental protocols on the "solubility of HCH isomer in imidazoles + AlCl3" and “reactions in CO2” had been submitted to the management of Farbwerke HOECHST in December 1950 with the request to pursue this field systematically in any way possible.
The replacement of organic solvents by low-melting, non-aqueous inorganic-organic solvents that are suitable for regeneration opens a wide experimental field which the author was unfortunately unable to pursue because of time constraints.
The correspondence in this matter exchanged in 1949 with Prof. Dr. G. Rienäcker, Director of the Institute of Inorganic Chemistry of the Rostock University, had been attached to the manuscript (which has the function of a memorandum) to emphasise the significance of this new direction of work; cf. re 3)
Unfortunately, this proposal backed by two lectures on December 15, 1952, was not taken up by Farbwerke HOECHST [lecture held on the occasion of a visit to the plant].
It is suggested to read the summary of the results on "ionic liquids" from the 90s in Chemical Reviews by Th. Welton [Chem. Rev. 1999, 2071 - 2083].
G) Regarding the visits
to
As mentioned under "re 14)", the author started travelling to
Brazil for one or two months at least once a year from 1964 onwards at the
request of UFSM to build up and organize a Chemical
Central Institute with all chemical branches in Santa Maria, Rio Grande do
Sul and to run this as a Coordinating Director after it opened in 1973, all at
the expense of the Brazilian government.
This was to continue for more than 20 years. This also applied for all travel within
During all the time the author worked in
Another fringe benefit was à la carte dining at the best restaurant of the town run by a Portuguese, again at no expense whatsoever to the author. An "Underberg" was served for disinfection purposes at the beginning of each meal.
These descriptions of the one- to two-months sojourns in
They exude a cheerfulness
which becomes catching after a while.
This is well characterised by two proverbs "God is a Brazilian; he will
put things right" or "Da un jetinho" (There is always a
knack"). This good mood always stayed with me for a good while after I
returned to
One must exercise great care when criticising anything, because Brazilians are very sensitive, but one quickly adjusts to this. The same applies for the topics of punctuality and scheduling appointments. "Yes" in the Portuguese language is "si", but often "pois não" is often used in the same sense.
Illustration 15:
Photograph of the author's collaborator of many years, W. Pollack (right-hand side: personal assistant, secretary, translator) and R. Machado (left-hand side: chief aide at the vice-chancellor's office).
Photograph 
Mr. Pollack was also the go-between with the university printers. He
arranged for the translation of all lecture notes [34] and the production of
the slides required for the author's lectures. He was also in charge of the 30
diagrams [Plates of large scale 1x2 to 2x3 meters] produced in
H) “Correspondenze dall’estero (dalla Germania)” per la
Rivista „La Chimica e l’Industria“, Milano
The editor of the above-mentioned Italian technical
publication, Professor Dr. A. Coppadoro, made the author an offer to become a correspondent based in
Here are a few quotations of the many articles sent to the editor of "La Chimica e l’Industria" sent over 14 years: one example in Plate 12 (next page but one)
La Chimica e l’Industria:
…38, 654 – 655 (1956) 41, 104 – 106 (1959)
39, 145 – 147 (1957) 41, 170 – 172 (1959)
39, 424 – 425 (1957) 41, 257 – 259 (1959)
39, 536 (1957) 41, 364 – 365 (1959)
39, 762 – 763 (1957) 41, 714 – 715 (1959)
39, 798 (1957) 41, 845 – 846 (1959)
40, 78 – 79 (1958) 41, 935 – 936 (1959)
40, 243 – 244 (1958) 41, 1043 (1959)
40, 325 – 326 (1958) 42, 1187 – 1188 (1960)
40, 605 (1958) 43, 100 – 102 (1961)
40, 799 – 800 (1958) 43, 1348 – 1349 (1961)
40, 965 – 966 (1958) 44, 138 – 139 (1962) … ecc.
This cooperation with the important Italian technical
publication LA CHIMICA E L’INDUSTRIA «Giornale di Chimica Industriale ed Applicata»
and «L’Industria Chimica», Milano, had the following history: In September
1940, the author had sent an original manuscript to the editor of GAZZETTA
CHIMICA ITALIANA,
Table 3: Start of the cooperation of many years with the following scientists and institutions - w/o Lohmann [re 5)], Matter [re 4b)], Mariano [re 14)]
First contakt country scientists institutions time
1948 Japan Professor Dr. S. Takei Institute of Agricultural
Professor Dr. M.
Nakajima Chemistry, University Kyoto
1948 Japan Professor Dr. Y. Morino Chemical Institute, University
Dr. J. Shimozawa Tokyo
1948 Brasil Professor Dr. R. Wasicky, Pharmaceutical Institute, Universi-
Senior dade S. Paulo, SP 1955 – 1972
1962 Brasil Professor Dr. R. Wasicky, Biochemical Institute, Universidade
Senior S.Maria, Santa Maria, RS 1960 – 1980
1949 Italy Professor Dr. D. Marotta Instituto Superiore di Sanitá, Roma 1949 – 1980
Professor Dr. M.E. Alessandrini
1950 Italy Professor Dr. G. Natta Università Técnica, Milano 1951 – 1970
Professor D. A. Coppadoro Milano, Redazione „LA CHIMICA e
L’INDUSTRIA“ 1954 – 1968
1950 Sweden Chemische Industrie Skoghallverken, Skoghall 1951 – 1958
1948 Germany Direktor Dr. F. Scherer, Farbwerke HOECHST AG 1949 – 1985
Professor Dr. Winnacker
1949 Germany Direktor Dr. A. Peters, Bergwerke AG, Essen 1949 – 1958
Dr. H. Brodkorb 1949 – 1958
1950 USA Direktor Dr. W.B. Ligett Ethyl-Corporation, Detroit, USA 1950 – 1965
Dr. H. Klopfer 1948 – 1975
1950 Italy Direktor Dr. Paesano Montecatini, Milano 1951 – 1956
Plate 12: Esempio per „CORRISPONDENZE DALL‘ ESTERO
dalla Germania“ nella Rivista: LA CHIMICA E L’INDUSTRIA 40, 605 (1958)

Die in zweiten Artikel formulierten Verbindungen sind „LOST-Analoge“. Experimentelles über die Grundverbindung ß,ß-dichlor-diethylsulfid, in PROJEKT XVIII: „Permeation problems“ [1].
J) Start and development
of hexachlorocyclopentadiene chemistry
in
Overview in chronological order 1930-45:
1930: F. Strauss, L. Kollek, W. Heyn,
Preparation of C5Cl6 from cyclopentadien & hypochlorite
Ber dtsch chem Ges 1930, 63,1868 (orientational experiments only) - Prof. Strauss died in 1930.
1941: R. Riemschneider,
Preparation of C5Cl6 from C5H6 and hypochlorite
When studying chemistry in
Just during the preparation from cyclopentadiene and hypochlorite a reaction between C5Cl6 and the starting material C5H6 seemed to take place, forming waxy residues in more or less large amounts. Further investigations of the waxy products were projected as soon as possible: Table 4
Table 4 : The possible reactions taken into consideration concerning the preparation mentioned above
Main reactions:
1) C5H6 + 6 OCl’ + x OH’ = C5Cl6 + (6 + x) OH’
2) C5H6 + 5 OCl’ + x OH’ = C5HCl5 + (5 + x) OH’
Follow reactions:
3) C5Cl6 + C5H6 = C10H6Cl6 : C5Cl6 as diene
4) C5H6 + C5Cl6 = C10H6Cl6 : C5H6 as diene
5) C5HCl5 + C5H6 = C10H7Cl5 : C5HCl5 as diene
6) C5H6 + C5HCl5 = C10H7Cl5 : C5H6 as diene
7) C5H6 + C5H6 = C10H12 : dimere
8) C5Cl6 + C5Cl6 = C10Cl12 : dimere[iii]
9) Polymerisations of monomers
Reactions 1-8 are realized and described in PROJ.XI and XVII.
Also there report about the following reactions:
C5Cl6 + C10H6Cl6 from reaction 4): C15H6Cl12
C5HCl5 + C10H6Cl6 from reaction 4): C15H7Cl11
C5Cl6 + C10H7Cl5 from reaction 6): C15H7Cl11
C5HCl5 + C10H7Cl7 from reaction 6): C15H8Cl12
1942 R Riemschneider, F. Maaz
Reaction of C5H6 and hypochlorite and investigations of the waxy by-product (follow-product),
lab
report June 1942, experiments carried out
in leisure time in the laboratory of “Schule Base” in
Results : The “wax” contained the C5 components [ C5Cl6, C5H6] in the ratio 1:1 of the summary formula (C10H6Cl6)n [ n = 1, if polymerism excluded]
1942 R Riemschneider
Investigations about the constitution and properties
of the waxy product “C10H6Cl6”
(I), resulting during the preparation of C5Cl6 +
OCl’ [according to prescriptions of 1941
(
Preliminary report June 1942 (secreted)
Results: In series experiments of C5Cl6-preparations, experimentating with a surplus of C5H6, the yield of the waxy part I was increased.
First hints as to insecticidal effect (flies) of I: Sometimes we found a lot of dead flies on or near the lab table.
Treatment of I (melting within a certain range) in CCl4 with Cl2-gas resulted in an oil (II), containing 2 Cl more than I. II was more effective than I (flies).
Experiments concerning C5Cl6-preparation
and C5Cl6chemistry continued in Prag and
1943 R. Riemschneider
“Comparison of “explosive” properties of C2Cl2 and C5Cl6”
Lecture given 8 Febr 1943 in Prag in the German Army Explosives and Warfare Agents Research Establishment, “Heeresforschungsinstitut für Explosivstoffe”.
Lab report of these experiments issued in 1943 in the mentioned Institute in charge of military service.
Results: C5Cl6 did not come up to the expectations of the direction board of the institute: C5Cl6 was – contrary to C2Cl2 - not qualified as explosive,
but it showed unexpectately – corresponding to our own earlier observations (see above) – high chemical reactivity in general with unsaturated compounds of all kind.
1943 R Riemschneider (lecturer), A Kühnl, O Schmidt
Hexachlorocylopentadiene, C5Cl6: 2 lectures
1: “The action of sodium hypochlorite on cylopentadiene: hexachlorocyclopentadiene and higher-melting reaction products” (Table 4)
Lecture given on 15 Feb 43, 20 p
2: “Properties and reactions of C5Cl6”
Lecture given on 15 Mar 43
in German Army Explosives and Warfare Agents Research Establishment in
The “example” of dichloroacetylene, C2Cl2,
made it seem appropriate to test if C5Cl6 was also suited
for an explosive or warfare agent. Result: negative (see experiments described
earlier, also carried out in
In this lecture, the author did not mention his observations from 1942 about the insecticidal activity of the waxy residues and its chlorination products.
1943 R Riemschneider
New waxy “DIELS-ALDER-adducts”
a) isolated from C5Cl6-preparations as “by-product” (Table 4)
b) prepared from pure C5Cl6 + C5H6 (in an exotherm reaction)
Continuation of the experiments carried out in 1942 in
Lab
reports, April 1943, 16 p, RUHRÖL,
1943 R Riemschneider
Constitution and properties of wax-like side products, and their properties, obtained when preparing hexachlorocyclopentadiene from cyclopentadiene and hypochlorite. In fact new DIELS-ADLER adducts
Lab
reports, May 43, 30 p, RUHRÖL,
1943/ R Riemschneider
1944 On using hexachlorocyclopentadiene, C5Cl6, as starting material for “synthesis of chlorine compounds with new spatial distribution of chlorine”: OET group (later: DIENE group)
Lab reports, Dec 43, 9 p (secreted)
Experiments
conducted at RUHRÖL GmbH,
1943/ R Riemschneider
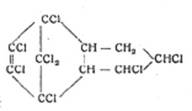
1945 On the remarkable properties of post-chlorinated adducts from C5Cl6and cyclopentadiene, C10H6Cl6 – M 410 identified as octachloroendomethylenetetrahydrohydrindene (OET),
![]() C10H6Cl8 (mol wt 410):
C10H6Cl6 + Cl2 C10H6Cl8
C10H6Cl8 (mol wt 410):
C10H6Cl6 + Cl2 C10H6Cl8
Lab reports 1944 and to Mar 45. Experiments conducted at RUHRÖL GmbH and in Inst of Hyg, Univ of Jena
Further public ations in PROJ XI and XVII in [1].
At the end of the 40ies Riemschneider visited[iv] the discoverers of the DIEN-synthesis profs Dres Dr.K.Alder, Cologne, and O.P.H. Diels, Kiel, - both Nobel prize winners - to inform them personally about his discovery concerning the unexpected C5Cl6-chemistry and especially to reach consent to “leave this research field to him”.
CN-analogues C5(CN)6, (CN)2C=C(CN)2
It had been the obvious thing to try - after our discovery of the unexpected high reactivity of C5Cl6 – to check the reaction of high substituted CN-analogues in DIEN synthesis, i.e. hexacyanocyclopentadien, C5(CN)6 (I) and tetracyanoethylene, (CN)2C=C(CN)2 (II).
In the case of II we were already in 1949 lucky enough to find out that II reacted as philodien: PROJ I: there ref (34,102). The synthesis of I remaines still open, today.
In the case of II the author thought it advisable to contact once more the DIEN “pope” professor Alder, informing and asking him to leave this field for a certain time to the author. -
Together with Dipl.Ing.O.Matter, Vitznau, the author made a Swiss patent application.
1944 R Riemschneider (lecturer)
Lecture 1: "The spatial distribution of the Cl-atoms of Gesarol's active ingredient (later DDT) and of halogenated adducts from hexachlorocyclopentadiene and cyclopentadiene: DIENE group insecticides, eg M 410 (C10H6Cl8) as well as M 393, M 377, M 344"
20 min lecture in Inst of Hyg at Univ of Jena on 15 Dec 44, invited by Prof Dr H Schloßberger (chair). Present: Profs Dr H Bredereck (org chem), Dr H Brintzinger (tech chem) [at instigation of Dr G R Schultze, Braunschweig Tech Coll, author's doctoral supervisor], Dr F Hein (anorg chem), Dr H Keller (phar-ma) and Dr Scheffler, dean of Fac of Math and Nat Sc
Lecture 2: "The naturally occurring insect toxin cantharidin as a model substance for developing synthetic insecticides with a five-membered ring structure, eg M 410 "
given in colloquium of Inst of Hyg, Univ of Jena on 10 Dec 44, chair Prof Dr H Schloßberger
Cantharidin as model substance for insecticides with five-membered ring structure, eg M 410
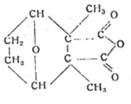
Cantharidin M 410
1945 R Riemschneider
“A new type of insecticidal halogen compounds, starting from C5Cl6 – after first tests, very promising: M 410” Lecture given on 31 Jan 45 in Inst of Pharmaceutics, Univ of Jena
Insecticidal activity of M 410 on Musca domestica, Drosophila melanogaster, Calandra granaria, Blatta orientalis and five other insects greater than DDT
The author wants to take this opportunity to thank
Prof. Dr. Pflugfelder, Zoological institute of
This report about the hexachlorocyclopentadien chemistry was handed over in Dec 1947 to Farbwerke HOECHST for the Patent Department and for the Department of Plant Protection – together with some material collected as „Mitteilungen des Physiologisch-chemischen Instituts der Universität Berlin“.
More details about hexachlorocyclopentadien chemistry in PROJ XI [1] and XVII [39].
According to the opinion and justification of
- Director Dr Scherer (HOECHST),
- Nobelprice winners profs Dres A. Alder and H. Diels,
A. Butenandt and Richard Kuhn
the discovery of the unexpected high reactivity of hexachlorocyclopentadiene and the insecticidal properties of pertinent adducts are entirely due to Riemschneider M 410, C10H6Cl8; cf SPECIAL PART J).
In his lawsuit between the HYMAN Company of Denver and VELSICOL Company of Chicago HYMAN asked Riemschneider for proof that he had had discovered M 410 (C5Cl6 based) before him[v] and VELSICOL. However, this only succeded in part.
Privately speaking HYMAN was convinced; cf. ADDENDUM next page.
The two directions of work „C5Cl6 chemistry”
(Riemschneider, Hyman) and “metalorganic
Since 1968 there was a contact between the Nobel committee and Riemschneider as one can see from a copy of the letter by the committee inviting Riemschneider to propose a candidate.

In USA: The lawsuit between VELSICOL-Corporation and HYMAN -
Company concerning CHLORDANE, ALDRIN and DIELDRIN (Hyman’s two own later developments[vi]) went through tree instances. Results: VELSICOL was permitted to produce CHLORDANE and SHELL took over the production of Hyman’s ALDRIN and DIELDRIN; cf. endnote 26.
In
Regarding M 410 and analogues there were the following patents (cited in http://www.bwwsociety.org/journal/html/pestcontrol.htm 2005):
R Riemschneider (inventor), O Matter (applicant)
Two patent applications entitled "Pest control agents"
1) Insecticides based on hexachlorocyclopentadiene (C5Cl6) adducts from cyclo-pentadiene and hypochlorite and post-chlorination of chlorine number 6 [or 5] to 7 or 9 [or 6 to 7]" If pentachlorocyclopentadiene forms as a by-product while preparing C5Cl6 by hypochlorite-chlorination
2) "Insecticides based on adducts from tetrachlorodifluorocyclo-pentadiene (C5Cl4F2) and cyclopentadiene and post-chlorination on chlorine number 6: C10H6Cl6F2".
Due to the circumstances of the time, it was only possible to apply for these patents in 26 May and June 47 with the help of Swiss friends, namely Dipl Ing O Matter [naming Dr R Riemschneider as inventor], after the author had left the Soviet occupation zone for Berlin. The applications were prepared from autumn 46 by coded correspondence* between Jena and Vitznau, Switzerland [mail censored by occupying powers] Only the general formulas A and B were used in application 1 for the insecticidal and / or fungicidal active agents of the pest control agents to be patented to avoid a collision with the structural formulas,
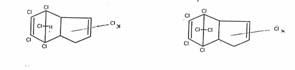
A B
already worked out and published, of the chlorine compounds C10H6Cl8 (M 410) [or C10H7Cl7] and the adduct precursors C10H6Cl6 [or C10H7Cl5], known to be active.
Patent claim to application 1 (26 May 1947):
Pest control agents, containing chorinated hydrocarbons of the general formulation A and B, that are obtained as follows: Chlorination of cyclopentadiene with alkaline hypochlorite solution [1 : 6 to 1 : 7], initially in the cold, then at temperatures up to 60°C and subsequent direct chlorination of the organic phase of the reaction mixture in the presence of UV light or other catalyzers, preferably post-chlorinated on a chlorine number of a reaction mixture from 8 or 7, related to C10.
A patent application by Farbwerke HOECHST became known on 06 Sept 56 [F 8439 Iva/45 I, entitled Pest Control Agents, applied for on 26 Feb 52, ie 5 years after receipt of the text of the patent applications of 26 May and in June 47] in which the active agents formulated by the author in the patent claim to application 1 were claimed by reason of tests by Hoechst's Plant Protection. The chemical page of the specification corresponds exactly to the application text the author had made available to the management 5 years before the application. However, so as not to endanger HOECHST's application, the author agreed in 1955 that literature as well as his papers on hypochlorination not be quoted after the fact. The author forwent being named as the inventor as the experimental part of the application by and large only contained biological data and as it was already clear at the time that - in view of developments in America: aldrin, dieldrin - the object of the patent would hardly become significant [39].
K) Explosives
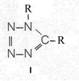
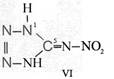
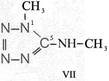
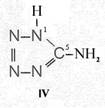
On the occasion of the presentation at the OKH in Berlin in December 1942 and after his two lectures (819a, b) in [1], also in Berlin at Jebenstrasse, the author had suggested to Major Kassuba who chaired the presentation and gave the introductory address to the lectures to use a class of compounds rich in N such as tetrazoles, pentazoles, their amino derivatives such as diaminotetrazole (V) and their salts (e.g. V-perchlorates, V-azides), azotetrazolates, etc. in combination with compounds containing NO2 groups as high-energy compounds.
In this respect, see
Formula Summary I: "Projected high-energy compounds of the class of representatives in N: N3 to N10 (1942)"; cf. also [1]: (819b) there para. g).
The starting point for these proposals were the publications of J.
Thiele (1892 - 98) and R. Stolle (1933) [cited
under 4b)Oskar Matter]. As already mentioned, first experiments were
conducted in
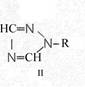
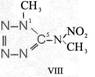
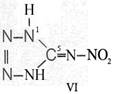
Formula Summaries II: "Nitration of 5-amino-tetrazole (IV) and IV-derivatives such as VII to VIII" and
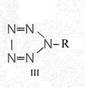
Formula Summary III: "1,5-Diamino-tetrazole (V) starting from thiosemicarbazide" [second synthesis of V appears to be more advantageous]
Formula Summary IV: Azotetrazolate-salts.
These experiments were then continued
together with engineer O. Matter in
The combination of V or V-derivatives with trinitro compounds and the use of azotetrazolate salts (IX in Formula Summary I) had yielded excellent results regarding the characteristics required of explosives in 1954: Experiments carried out by Matter at his own explosion site on Vierwaldstätter lake (unpublished, secreted - even after Matter's death). He developed data regarding the heat of explosion and detonation (P, D), stability (impact, heat, friction, electrostatics). During his visit in 1954, Mr. Matter entrusted the author both with all the data and the information regarding the compounds used.
Formula Summary I1)
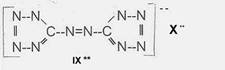
Projected high-energy compounds of the class of representatives in N : N3 to N10 (presented to the OKH in 1942: I-V; IX-XI)2)
Amino-I derivates: IV, V
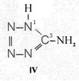
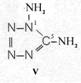
IV- derivates: VI to VIII
azotetrazolate-salts: IX
biuret-nitration product, i.e.: X
O2N-NH-CO-NH-CO-NH-NO2 X
Diamino-dinitro-ethylene: XI
(H2N)2-C=C-(NO2)2 XI
1) with letter from Dec 12th, 1956 sent to the
“Bundesministerium für Verteidigung” in
2) starting from J. Thiele, Liebigs Ann.Chem. 270,1 (1892) and R. Stolle, H. Netz, O. Kramer, S. Rotschild, O. Schick, J. prakt.Chem. 138, 1 (1933).
Formula Summary II: Nitration of 5-amino-tetrazol (IV)
and IV-derivates i. e. VII to VIII
(experiments carried out in Prag 1943)
HNO3![]()
![]()
(CH3)2SO4
CH3J
HNO3![]()
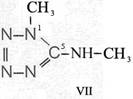
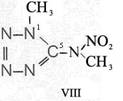
IV synthesized according to J. Thiele
Formula Summary III: 1,5-Diamino-tetrazol (V) starting from Thiosemicarbazid (experiments started in spring 1943 in Prague, continued in Switzerland 1953 and later in Brasil, from 1970 on)
N3H![]()
![]()
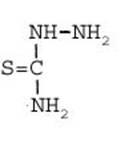
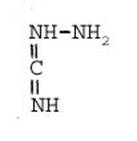
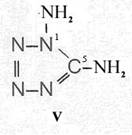
Thiosemicarbazide was converted into 1,5-diamino-tetrazole (V) with PbO and NaN3 in a CO2 atmosphere via an intermediate with HN3.
The Pb azide resulting as a side product causes problems and is therefore unsuitable for industrial use.
Another method of production for I is desirable: I forms salts with perchloric acid and hydrogen azide.
1972:
New synthesis of V :
Diazo treatment of diaminoguanodinium-halogenids in a narrow pH range 7,6-8,2 at room temperture (30-35° C).
Reaction also dangerous: azid formation if pH not taken into consideration. To find out the best pH range we carried out 14 series experiments behind a protecting iron plate because of the expected azid formation.
The author owes thank to drs M M Faria and F R Pesserl for co-operation and their courage - 8 explosions!
Experimental Part to 1,5-diamino-tetrazol (V) experiments
R.Riemschneider,1943 and R.Riemschneider,O.Matter, 1953
1,5-Diamino-tetrazol (V) and V-nitrat, V-perchlorat, V-azid
Patentapplication O.Matter, inventor: Riemschneider und Matter.
V was prepared in accordance with R. Stolle et al., J. Prakt. Chem. 1933, 138, 1. Because of the risk of explosion, very small batches were used to obtain sufficient V.
Preparation of 1,5-diamino tetrazole ( V):
55 ml of an aqueous solution containing 4.5 g of diamino guadininium chloride and 1.5 ml of concentrated HCl are prepared under an exhaust hood behind an iron plate. Diazotisation with sodium nitrite is carried out under an N2 flow at 2 to 4°C: 20 ml of nitrite solution containing 2.5 g of NaNO3 are added dropwise with strict observation of the required temperature. After 45 minutes, the pH value of the reaction mixture is adjusted to 8.0 to 8.2 with Na2CO3 and stirred slowly at 45°C for 25 minutes, still under nitrogen. After that, the N2 flow is concentrated in vacuo until dry (keep a safe distance!). After cooling, repeated extraction with a little ethyl alcohol at 60°C and recrystallisation from water as before is carried out, V mp 185°C (low yield).
A total of 19 serial experiments were required to determine the optimal conditions for preparing V. In the present case, we benefited from the experience with V gained as early as 1943 and in the 1950s. After a heavy explosion occurred during the second serial experiments, we worked with grip arms wherever possible, just as in our experiments with dichloroacetylene and monochloroacetylene [R. Riemschneider, A. Brendel, Liebigs Ann. Chem. 640, 1 - 15 (1961)] und in the nitration of meta-dinitrobenzene to form trinitrobenzene:
R. Riemschneider, A. Kühnl (1943).
V-nitrate
The formula to prepare is the same as indicated in the patent application of 1953. Observing the precautions specified above:
4 g of fresh prepared V are added to 3,0 ml of 66% HNO3 at 20° C in N2-stream and very slowly heated, then under cooling given to the clear solution ethylether in amount of 25-35 ml. The precipitated cristals are filtered off and washed with ether, dried over P2O5 after cristallisation from ethanol: 2,5g V-nitrate, mp 135-138°C.
V-perchlorate:
Observing the above precautions and working with grip arms, 2 g of V are dissolved in 25 ml of high-purity methyl alcohol. 1.5 g of concentrated perchloric acid are added gradually with slow stirring, still under nitrogen. After reacting at 20°C for 1.5 hours, the mixture is extracted with ethyl ether, mp 97-98°C.
V-azide: high explosive, difficult to handle
|
V-preparation |
CH4N6 |
100,07 |
calc. |
C 12,0%, H 4,0%, N 84,0% |
|
|
|
|
found |
C 12,2% H 4,0% N 83,8% |
|
V-nitrate |
CH5N703 |
163,1 |
calc. |
C 7,4% H 3,1% N 60,1% |
|
|
|
|
found |
C 7,3% H 3,0% N 60,0% |
|
V-perchlorate |
CH5N6Cl04 |
200,5 |
Calc. |
C 5,9% H 2,5% N 41,9% |
|
|
|
|
found |
C 5,8% H 2,9% N 41,6% |
The explosive characteristics of these compounds prepared in Vitznau and
Result: V-nitrate, V-perchlorate, and V-azide are comparable with TNT.
Detailed experimental data are set forth in specification of the Swiss Patent applied for by O. Matter, inventors: R. Riemschneider and O. Matter. The letter from Mr. Matter to the author in December 1954 expresses delight that the three V-salts have explosive characteristics comparable with TNT.
More details in the essay “High Energy Compounds – Explosives“ later to be published in this JOURNAL with the ten Formula Summaries A - J plus Experimental part, 40pages.
Summary
The author considers his activities in chemistry in
This paper gives an impression how politics influenced the author’s life and activities - more or less negatively – and his “fights” against politics - looking for ways-out. All resistances and intrigues have been got over, and in the end chemistry won.
Acknowledgements:
The presented results, >1400 references in [1], have been be possible only with the help of many, many co-workers like students, candidates of examination papers, collegues, and employees in Germany, Japan and Brazil, named in [1]. The author wants to thank all of them herewith once more.- The long list of Diplomanden, Doktoranden and Lehramtskandidaten incl. titles of the papers, presented in different universities (HU, FU, UFSM, and together with collegues in others) will be published separately elswhere, corresponding to the XXVI projects and other data, not regarded in [1].
It is planned to publish the above mentioned extensive co-worker list together with the author’s patents and expert witnesses, every title containing a summary of the main results, the patent claims and reasons for expert opinion.
ADDENDUM - re [32, 33]:
Prof. L. Pauling and the ortho-molecular medicine[vii] (definition in last paragraphs)
Citation [32] goes into discussions of the author with Professor Linus Pauling [double Nobel Prize winner: Chemistry and Peace], namely discussions from the years 1969 - 72 primarily dealing with the vitamin C-complex [L-ascorbic acid plus dehydro-ascorbic acid].
It is well known that Prof. Pauling reported "Successes in the field of cancer prevention" on the occasion of a convention of Nobel Prize winners in Lindau; these successes are due to the oral intake of vitamin C in doses significantly exceeding those needed every day. In private talks, the author had voiced his concerns regarding high vitamin C quantities on the basis of his own toxicological experiments with rats: The oxalate concentrations in the rats' blood plasma increased after the oral application of 20 mg of vitamin C per kg (determined DL50 per rat: 12 g/kg). The author had set forth his considerations regarding the following points:
- "Stability of hydride ions in aqueous solutions"
- "Organic compounds like vitamin C-complex, vitamin E-complex (NADH, flavonoids) acting as “transport vessel” for hydride ions[viii] (H atoms, respectively)"
- "radical-scavenger effect of hydride-ions" [33]
He thus asked Prof. Pauling whether tests had been carried out "how tumours react to vitamin C applied intravenously, because the advantages vis-à-vis high oral daily administration are obvious"; when orally taken only small amounts of vitamin C will get into the blood. The author had not only cancer prevention in mind but also cancer treatment. Paulings answer to the above mentioned question was: "Not as far as I know. Also Dr. Iwan Cameron applied Vitamin C only orally (pure powder teaspoonwise) in his cancer prevention experiments. - Keep me informed about your activities!"
In the 1970ies, together with his candidate for a senior university post, Dr.Lipp[ix], of the Robert-Koch-Institute, Berlin-Dahlem, the author was able to show in experiments with mice and rats that vitamin C applied intravenously inhibits the growth of ascites cells. Injected Vitamin C inhibited the growth of stomach- and pancreas tumor cells which were generated before in rats. In some experiments we had success with dehydracet acid [x], too
In parallel running cell culture experiments, vitamin C - also given in comparatively high doses - had a negative influence on the growth of several cancer cell types, up to 30-40% growth inhibition, but it did not injure healty cells [unpublished at the request of Prof. Henneberg, director of the Robert-Koch-Institute, to gain time for clinical experiments. Professor Pauling agreed with this].
The first clinical experiments with intravenous vitamin C - intending cancer “chemotherapy” - were not carried out until 1977 [xi]:
- at Klinikum-Westend,
- later at the university teaching hospital of UFSM, Santa Maria, RS, Brazil, under Prof. José Mariano da Rocha Filho (former rector of UFSM).
Only when comparatively high doses of vitamin C were injected, some success was shown in cases of cancer of the pancreas and the prostate.
At the time, it was not possible to continue the clinical experiments:
- Prof. Gerhartz was retired; once again, the "democratised“ Free University failed.
- Professor Mariano was not a specialist in oncology and primarily had to devote his time to other duties.
- The author was deprived of any possibility to continue this important research at the Free University xi.
In
- 1) the vitamin C complex under varied conditions,
- 2) compounds constitutionally related to the C- and E-complex
- 3) fruit and vegetable juices in suction flasks for drinking water for a "therapy with hydride ions" for at least three months [33],
- 4) combination of 3) with 1) respectively 2) with 1) [33]
These results were communicated to Prof. Pauling in the hope that he would arrange clinical tests. Unfortunately, this did not come about, because Pauling died in 1994.
Conclusion:
Without the much criticised extensive "vitamin C experiments for cancer prevention" of Prof. Pauling (people said the oral dose was too high) and without Pauling's activities, ortho-molecular medicinevii would certainly not have been developed in such a prominent way.
Without such suggestions, the author would not have developed
"synthetic organ and plant extracts" (EP 0 552 616 B1) up to the
production-line stage and organised their application in
Nor would the author have arranged intravenous vit C-complex applications in hospitals based on his own cell culture experiments without the encouragement of Prof. Pauling.
Without such encouragement and without the first positive clinical experimental results the author would not have carried out additional cell culture tests starting from the vit C-complex as described in the previous paragraph.
Definition of “ortho-molecular medicine” according to R.Rowghani, cited in the endnotes:
“L. Pauling geht bei der menschlichen Gesundheit davon aus, dass man in den gesamten Körperzellen „gut angeordnete Moleküle“ vorfindet, ein zu erreichender Zustand, welchen Pauling in den 60er Jahren ORTHOMOLEKULAR nennt (orthos = richtig; molekular = Moleküle betreffend). So enstand die mittlerweile bekannt gewordene und bei Ärzten angewandte ORTHOMOLEKULARE HEILKUNDE, die besagt, dass der Mensch erkrankt, wenn er nicht mehr in den Körperzellen die lebensnotwendigen Moleküle aufweist, was zuerst zu Fehl-funktionen und schliesslich zu bisweilen schweren Krankheiten führt.“
According to the author’s opinion [33] Norman W. Walker had achieved pioneerwork in this field; and as result of living in this way:
- Walker himself reached an age of 116 years.
- Pauling reached an age of 93 years.
ADDENDUM
to „RONGO“ refering to Plate 6 (Part II)
The German translation of the Confucius quotation mentioned in Plate 6
was superimposed in German language during the TV broadcast of the opening
ceremony of the Olympic Games in
During the 5-hour ceremony, significant phases of the 5000 year history
of China were presented, for example the outstanding invention of gun powder,
the production of paper, of typefaces, print, compass, Chinese legendary
creatures (dragons), Chinese opera. The choreography with bamboo scrolls
presented by 3000 Confucius students (with
the words from the work "RONGO" superimposed - same
words calligraphed by Professor Morino for the author in Plate 6) was most
impressive. To make such a presentation successful, all of the participants had
to have an excellent memory and ability of cooperation. There were also choreographies
involving 2008 Kung-Fu students and others, always presenting 2008
participants. On the whole, 15,000 different costumes were used, including the
traditional costumes of the 57 different nations of
The gigantic opening ceremony was complemented by breath-taking fireworks any many colourful light effects with huge projections.
Colourful fireworks went up not only in the air and not only around the
stadium - no, these extended over half of
The ARD-TV commentary did not do justice to this fantastic event; rather, it showed that the commentator did not know the theme and, like the closing interview, contained anti-Chinese slogans.
Most of the major states had sent their presidents or high-ranking representatives to the Middle Kingdom; unfortunately, no important German politician was present.
The performance given by the Chinese did not surprise the author in the
least. In contacts with Chinese candidates for diplomas and doctorates and with
representatives of the Chinese industry over many years, the author was able to
see with his own eyes what these people can achieve. - Two outstanding
associates of the author in a university career of more than 50 years came from
BIBLIOGRAPHY
[1] R.Riemschneider,
“Re-reading – 66 years chemistry” (in preparation)
[2] R.Riemschneider,
Bonded to Compounds –
http://www.bwwsociety.org/journal/html/bcompounds.htm 2005
In this essay the author treated the subject „Yeast“ only briefly.
More details, especially about the author’s yeast patents, in the publication “Way out of the BSE-crisis” [38].
[3] VITA Riemschneider,
written from Prof.Dr.Heimo Reinitzer, president of the Hamburg Akademie der Wissenschaften, published in [1]
[4] R.Riemschneider,
Oxidation of ethylbenzene, o-,m-,p-diethylbenzene and o-ethylaceto-phenone with potassium permanganate in buffer solution:
o-diacetylbenzene - Permanganate oxidation in organic solvents.
Voluntary Year’s Paper in chemistry for graduation at
Summary of some results published 1947 as bulletin I
in series “Acylderivate cyclischer Verbindungen”, deposited on 12 Sept. 1940
with editors of GAZZ. CHIM. ITAL.,
[5] R.Riemschneider
Oxidation of DecalinTM (decahydronaphthalene) in acetic anhydride with potassium permanganate to cis-β-decalolacetate degree thesis, University of Leipzig, Sept.1941, 40 p; excerpt published in 2. Beih., 1. Erg.-Bd. Z. Pharmazie 1947, p 142
[6] R.Riemschneider
Zur Kenntnis der Kontakt-Insektizide I und II“,
2. und 9. Beiheft zur Zeitschrift „Die Pharmazie“ 1947 und 1949 Verlag Dr.Werner Saenger, Berlin(OST)
The largest part of the monograph "Knowledge of Contact
Insecticides II" comprising 150 printed pages was written during the time
of the Soviet blockade of Berlin(WEST): Berlin(WEST) was the official
designation in the West for the three West Sectors. In contrast, Westberlin was
written in one word starting with a "W" in the East . Instead of
Berlin(EAST), the name used there was “
Since electricity was available in the West Sectors of the city for only two to four hours a day during that time and was usually cut when needed, the author rented a room in Berlin(EAST) near the Institute [The Humboldt University, at the time the only university of Berlin, and therefore also the Physiological-Chemical Institute were located in the East Sector of the city] unofficially, so to speak. This also had the advantage that the author saved the time for the twice-daily Underground trip of one hour each and was able to use the laboratories longer (During the time of the blockage, the underground ran only until 6 p.m.). Moving completely to the East Sector was no option for obvious reasons.
[7] R.Riemschneider
Lecture „Molekulare Asymmetrie“, given on 28. 2.1948, cf also PROJEKT II in [1] and in [19]: stereochem.
[8] R.Riemschneider
„Material
für biochemische Einführungsvorlesungen“ (1969 1.Ed) 1986, 74 p, 4. Edition, W Hilke KG,
[9] Ministério da Educação e Cultura, UFSM, Centro de Estudos Basicos. “Inauguração do Istituto Central de Química, S.Maria , 24 de Agosto de 1973” , 16 p
“Autorga do Titulo Prof.honoris causa ao Prof.Randolph Riemschneider” 24 de Agosto de 1973”
[10a] R.Riemschneider (lecturer)
“Reactions in compressed CO2” - “Inorganic-organic solvents having a low melting point (polar ionic, non-aqueous solvents) Experimental data reports 1948 – 1952
Ms (leaf sheet) 1950, 15 pages + 14 pages experiments
2 lectures held at HOECHST on December 15, 1952 (secreted), Experiments in compressed CO2 continued 1965-2007
[10b] R.Riemschneider, F.R.Pesserl, D. Kirstein
Clathrates in compressed CO2 - Preparation of methane-ice (I) –
Investigation of the reaction between I and CO2-compressed
ms 1999, 18p (secreted)
Experiments carried out in 1998/99 in the labs of
BRASTONE,
[11a] R. Riemschneider (lecturer), J C Hilscher, E B Grabitz
“Chemistry of Polychloro-bicycloheptene-bis-hydroxymethyl and cyclic sulphite esters (THIODANE group)”
Lecture given Aug 61 in Inst of Agric Chem, Univ of Kyoto;
Ms 1960, 17 p, Diagrammatic representation of formulas in Pl 13 and 14: PROJ XI in [1]
[11b] R. Riemschneider, J. C. Hilscher, F.Franco, R.Schlepergrell, B.Götze, R.Remke, W.Ernst
Thiodan and related compounds
Bull I: The quantitative determinateion of thiodan,
Z.analyt Chemie 165, 278-280 (1959)
Bull II:
The stereochemistry of norbornene series cyclic sulphite esters and sulphoxides, Botyu Kagaku,
Bull III: Butane-2-diol-1,4-cyclosulphite and other sulphite ester,
Z.Naturforschg 15b, 552-554 (1960)
Bull IV: The state of research into of thiodan isomerism,
Z.Naturforschg 15b, 809-810 (1960)
further data in:
Chemistry of Polyhalocylopentadienes: The Chemistry on DIEN-group, World Review of Pest Control, Vol 2, 29-61 (1963)
http://www.bwwsociety.org/journal/html/pestcontrol.htm 2005, 16 p
[12] R. Riemschneider
INSTITUTO DE QUíMICA –
Ministério da Educação e Cultura, UFSM, 1965, 136 p: Plate 3a and b
[13] R. Riemschneider
Thalidomide - a remedy with two faces
http://www.bwwsociety.org/journal/html/thalidomide.htm 2004; 16p; cf [19]: thalidomide
[14a] R. Riemschneider (Vortragender), F.R. Pesserl, O. Matter,
T.J. Shimozawa
„η-1,2,3,4,5,6-Hexachlor-cyclohexane isolated from δ-oils“; (545) in [1]
Lecture from 15.9.1961, held at the Chemical Institute
Tokyo-University, chair: Prof. Dr. Y. Morino; PROJ. IX in [1]
[14b] Y. Morino, R. Riemschneider
Dipolmoment measurement of h-1,2,3,4,5,6-hexachloro-cyclohexane
ms 1961, 3 p; cf. [14a, 15]
[15] R. Riemschneider,
„7 stereoisomeric 1,2,3,4,5,6-Hexachloro-cyclohexanes“
Lecture, held on 15 Aug 1964, colloquium Uni Santa Maria, in Sala de Atos, Edificio sede da USM [Universidade de Santa Maria], Santa Maria, RS, Brasil.
ms, Aug 1964, 19 p (with Portuguese Summary), chair: Prof. Dr. José Mariano da Rocha Filho, Reitor USM; cf. Table 2 in SPECIAL PART E); zur Bezeichnungsweise: [16]
[16] R.Riemschneider:
Über „vereinfacht eben“ und „Sessel-Konfigurationen“ von Cyclohexansubstitutionsprodukten,
Z.Naturforschg. 10b, 605-613 (1955); vgl. auch Z.Naturforschg. 10b, 177-178 (1955)
[17] List of some Riemschneiders publications
issued in the Japanese periodical Botyu-Kagaku,
Chair configuration catalogue for C6H11X to C6X12
Botyu Kagaku,
Method for positional isomers of cyclohexane
Botyu Kagaku,
The spacial structure of hexachlorocyclopentadiene adducts
Botyu-Kagaku,
Pentachloro- and hexachloro-cyclopentadiene as philodienes
Botyu-Kagaku,
The stereochemistry of 7 cyclic sulfitic esters and 8 sulphoxides
Botyu-Kagaku,
[18a] R.Riemschneider
Cell line based organ material beats risk in animal organ extracts
http://www.vevy.com/relata issues, Articles 2001, 2p:
International Electronic Journal on Dermopharmacological Research, Dermopharmaceutical Technology and related cosmetic subjects
[18b] R.Riemschneider, M. Heisler
Porcine based organ extracts guarantee full substitution of bovine extracts
http://www.vevy.com/relata ,issues, Articles 2001, 2p; as [18a]
[18c] R.Riemschneider
Additivi cosmetici a base vegetale
[18d] R.Riemschneider
Two Notes on Progress in BSE-Crisis
http://www.bwwsociety.org/journal/html/bsicrisis.htm
The BWW Society/The Institute for the Advancement of positive Global Solutions 2003 , 4p
[18e] R.Riemschneider
Cytocatalyzer,
Cellryel, and Collaplant
http://www.bwwsociety.org/journal)html/cytocatalyzer.htm 2003
6 p; journal as [18d]
[18f] R. Riemschneider
Vegetal Placenta Extracts Substitute Animal Placenta Extracts
http://www.vevy.com/relata ,issues, Articles 2008, 7p:
International Electronic Journal on Dermopharmacological Research, Dermopharmaceutical Technology and related cosmetic subjects
[19] R.Riemschneider
some publications between 2004 and 2008 in:
http://www.bwwsociety.org/Journal/html/X.htm
|
X |
( subject ) |
year |
pages |
|
pestcontrol |
(chlorine insecticides) |
2005 |
16 |
|
thalidomide |
(Contergan, two faces) |
2004 |
15 |
|
bcompounds |
(heptachlorodioxanes) |
2005 |
7 |
|
heptachloro |
(heptachlorodioxanes) |
2006 |
11 |
|
isomerie |
(conversion-isomerie) |
2007 |
24 |
|
stereochem |
(cis-trans, axis-ring) |
2006 |
38 |
|
planttech |
(plant technology) |
2008 |
28 |
|
silanes |
(silanes) |
2007 |
22 |
|
viscosity |
(vT-behaviour, Cmax) |
2006 |
22 |
|
heptites |
(stereochemistry) |
2007 |
15 |
[20] R. Riemschneider, M. Spät, W. Rausch, E. Böttger
1,2,3,4,5,6-hexachloro-cyclohexane mp 88–89 °C (I) alias the real zeta-I-isomer e.e.a.e.a.a,
Mh.Chem. 84, 1068 – 1070 (1953): Tab 2
[21] R.Riemschneider
Bull I – VI, The so-called “zeta-hexachlorocyclohexane” resp
hexachlorocyclohexane, mp 146°C,
Z.Naturforschg. 5b, 246, 307; 6b, 48, 338, 41o (1950/51)
In these papers the author cleared up constitution
and configuration of the so-called zeta-isomer and identified it as 1,1,2,4,4,5.hexachloro-cyclohexane with chair configuration
1ea2e4ea5e(Cl).
For this compound the letter „ zeta” has to be cancelled. For this letter now belongs to the sixth isomer of the 1,2,3,4,5,6-hexachloro-cyclohexane series; see [20] and “zeta-Gezeter” in SPECIAL PART E).
[22a] R. Riemschneider, Y. Morino
"Calculation and determination of dipole moments of theoretically possible HCH isomers and generally of polyhalo-cyclohexanes"
Manuscript June 1953, 14 pages, written after a 5-hour
meeting with Prof. Morino in
[22b]
Dipolmoments of Polyhalocyclohexanes I,
Bull Chem. Soc. Japan 27, 177 – 180 (1954)
[22c] J.T. Shimozawa, Y. Morino, R. Riemschneider
Dipolmoments of Polyhalocyclohexanes III,
Bull Chem. Soc. Japan 28, 393 – 396 (1955)
[23] R Riemschneider
Mitt I: Derivati acetilici di combinazioni isocicliche: o-, m-, and
p-diacetil benzolo
Gazz Chim Italiana 77, 607 - 611 (1947), date of receipt 12 September 1940,
deposited in agreement with Prof C Weygand,
[24] R Riemschneider
„Tiocarbammati“
Lecture given to VII National Congress of Pure and
Applied Chemistry in
[25] R.Riemschneider
Lubricating oil-like hydrocarbons from products of pitch high-pressure hydrogen plant
Angew.Chemie B 19, 92-93 (1947)
[26] R.Riemschneider
Formulas and indices for rating viscosity-temperature
dependency in liquids
Bi-Monthly Journal Of BWW Society, Jan 2006, 22 p
www.bwwsociety.org/journal/html/viscosity.htm
[27] R.Riemschneider, Gg R.Schultze
„Aromatizing unsaturated aliphatic compounds“
Synopsis of the investigations in this field, covering from 1943 on; ref (232, 233, 235, 237,240-242) in PROJ III in [1]
[28] K.Schmidt, P.Kollek-Bös
Some Alkyl Thiocarbamates prepared from Thiocyanates by RIEMSCHNEDER’s reaction
J.Amer.Chem.Soc.1953, 6063; 1951, 5905;
cf also: H.Krauch, W.Kunz, F.Richter, Namensreaktionen der Organischen Chemie, Dr.A.Hüthig Verlag GmbH, Heidelberg 2001.
[29] R. Riemschneider
Heptachloro-1,4-dioxane mp 57°C - a compound of merely academic interest
Bi-Monthly Journal Of BWW Society, 2008 www.bwwsociety.org/journal/html/heptachloro2.htm [xii]
[30] R. Riemschneider
Bi-Monthly Journal Of BWW Society, 2008, 32 p
www.bwwsociety.org/journal/html/ pestcontrol2.htm [36]
[31] R.Riemschneider
Plant Technology – based on Chemistry, Botany, and Architecture,
published in Internet 2008, 28 p http://www.bwwsociety.org/journal/html/planttechnology,htm
[32] R.Riemschneider
Useful and questionable applications of several vitamins and anti-vitamins,
published in Internet 2008, 30 p
http://www.bwwsociety.org/journal/html/vitamins.htm
[33] R. Riemschneider
Nutritional Supplement by Hydrid Ions acting as Antioxidants and Hydrid Ions and H Atoms as "Energy Currency" for Living Systems
published in Internet 2004
http://www.bwwsociety.org/journal/html/hydrid.htm ;also published in [32]
[34] R. Riemschneider
Lecture notes in the Portuguese language (translated by W. Pollack), printed at the printing shop of UFSM:
"Biochemical Laboratory Courses I, II, III", all in all 180p
"Synthesis of biochemically relevant compounds" 80 p
"Organo-chemical preparations", 120 p
"Analytical organic chemistry", 80 p
"Collagene chemistry", 25 p
Manuals for all laboratory courses (each course with 20 to 50 participants) were supplied to prepare for practical experiments and to set up the apparatuses.
In order to get these laboratory courses going, all of the necessary chemicals and apparatuses were supplied from Germany by GTZ (Society for Technical Cooperation) as specified by the author, in quantities sufficient for 500 to 600 participants (DM 2 millions for the UFSM project).
[35] R.Riemschneider, M.M.Faria, F.R.Pesserl
A new synthesis of 1,5-diamino-tetrazol (V): Diazo treatment of diaminoguanodinium-halogenids (VI) in the pH range 7,6-8,2 at room temperature 30-35° C,
Lab report Nov 1973, 12 p, BRASTONE Company,
Remark: pH range important to avoid formation of dangerous azides.
[36] R. Riemschneider
Chlorine chemistry for pest control research - starting in the 1940s - with regard to the inventors and their "fights"
http://www.bwwsociety.org/journal/html/pestcontrol.htm 2005
complete article in APPENDIX to PROJ III 5,3;
[37] R. Riemschneider
ortho-Diacetylbenzene (o-Di) and some analogues in amino acid analytics and as marker in criminalistics - o-Di competitor of ninhydrin?
http://www.bwwsociety.org/journal/html/ortho.htm 2006
[38] R.Riemschneider
Way out of BSE-crisis
http://www.bwwwsociety.org/journal/html/bsiwayout.htm. 2008
The Bi-Monthly Journal of the BWW Society; cf. also the essay
http://www.bwwsociety.org/journal/html/bsicrisis.htm 2002
[39] R. Riemschneider
Chemistry of Polyhalocyclopentadienes, Bull XXXV: The Chemistry of the insectizides of the DIEN-group,
World Review of Pest Control, Vol 2 29-61 (1963).
[40] R.Riemschneider, H.Schlossberger
Discussion about the question: „Are there microorganisms which
can function as pollutant killer exterminating Cl-compounds?
Where to detect?“
15 Dec 1944 after the lecture of the author, held in colloquium Hyg. Inst., Jena Univ (SPECIAL PART J).
* eg: lines 1, 3, 5, 7 etc in one letter and lines 2, 4, 6, 8 etc in a second. After some letters were seized, we worked with three letters for one text, posted in different towns with differing senders
[i] In six publications under the title “The so-called zeta-hexachloro-cyclohexane” resp “Hexachlorocyclohexane mp146°C” the author cleared constitution and configuration of this C6H6Cl6 isomer: [21]
[ii] The oil reserves in known oil fields are
generally exploited only in an amount of 35 to 45 %. (After that, oil fields
are deemed practically depleted). With new technologies for an increase of
crude oil recovery, the yield can be improved, for example by pressing water
(as such or in the form of steam) or gas (CO2) into the fields
containing "residual oil". For example, this has been done in the
Fahud field, the Natih field, the Amal field (
[iii] not by DIEN synthesis
[iv] reisend unter schwierigen Bedingungen, wie meist in den ersten Jahren der Nachkriegszeit: In der Berliner Blockadezeit Flug nach Hannover, von dort nach Köln (Übernachten bei Bekannten) per Bahn; von Köln nach Kiel über Hamburg (Verwandte), zurück über Hannover und Flug nach Berlin.
[v] Auf Einladung von Hyman (HYMAN-Company) hat Verfasser am 15.5.1949 in Denver in einem round-table-Gespräch einen Vortrag zum Thema „Preparation and properties of M 410“ gehalten (Zitat in [1] ref (704). Dort erfuhr Verfasser, dass in der VELSICOL, Chicago, seinerzeit die Labor-Tagebücher täglich von zwei Personen unterschrieben worden sind, so dass Hyman sich nicht als alleiniger amerikanischer Erfinder beweisen konnte; vgl. auch nächste Endnote.
[vi] ALDRIN und DIELDRIN sind Hyman’s spätere Entwicklungen aus Denver; diese basieren jedoch ebenfalls auf der von der VELSICOL (aus Hyman’s Tätigkeit in Chicago) patentierten, auch für Chlordan gültigen Grundreaktion: C5Cl6 plus ungesättgte Verbindungen (die Verfasser schon 1941/3 entdeckt und 1943/45 geklärt hatte - leider zu einer Zeit, in der an eine Patentnahme nicht zu denken war, ebenso nicht wie in der ersten Nachkriegszeit); vgl.auch vorstehende Endnote
[vii] Ortho-molecular
medicine [orthomolekulare Heilkunde (ὀρθός = right)] founded by the American nutrition specialist Norman W. Walker [33], the
physician Max-Otto Bruker, and the biochemist Irwin Stone, taken over in 1966 by Pauling, which led to the Institute of
Science and Medicine founded later in
In 1954, Pauling was awarded the Nobel Prize for fundamental research carried out in the 1930s on the "nature of chemical bonds", and, in 1962, the Peace Nobel Prize for his "commitment against tests with nuclear weapons". Between 1935 and 1952, Pauling had achieved important findings in the field of biological molecules, just to mention: haemoglobin structure, helical protein structure (consequence: double helix by J. Watson, F. Crick, R. Franklin), development of antibodies.
Literature:
- A.Serafini, „L.Pauling – A Man and His Science“ Paragon House, New York 1989;
- R.Rowghani, „Wie lebt man länger und fühlt sich besser?“ Linde-Kultur-Verlag Berlin 2004.
[viii] Cf. also see plate 7 in ref [32]
[ix] During the years
1961 - 1967, Dr. R. Lipp prepared a post-doctorate paper on the "Toxicity
of antibiotics on plants" under the supervision of the author. This topic
was of great current interest at the time: In 1958, we were most surprised when
we found in cell culture experiments how toxic antibiotics are for plants. This
post-doctorate work was carried out at the Robert-Koch Institute and at the
Department of Biochemistry and at the
[x] Many years later, dehydracet acid years was interesting to produce resp to fortify “whitening effect” in cosmetic additives (EP 03 718 674.9 – PCT/EP 03/01874) from 24.02.2003.
[xi] The novel university law that came into effect in 1969 and was to make the FU "more democratic" made many scientific projects impossible or put an end to them in the years from 1969 to 1980:
- Due to a total restructuring and reorganisation such as abolishment of the departments, the deans, the academic senate and the vice-chancellor, the elimination of the institutes, their directors and removal of their authority to issue directives, strengthening of the administrative body (from 1969, a presidential administration with the power to lead the teaching staff by the nose); increase of the number of 150 working professors to approx. 850 by law in August 1969 (without increasing the university budget): the so-called "discount" or August professors.
- Due to the "interference of leftist students and so-called other service staff". These bodies had their spies everywhere, telephones were bugged, and relations with the industry were undesirable. There were "student working teams" without university lecturers, etc.
At the end of the 70’s, Professor Gerhartz did not dare touch "undisclosed research projects". The author had received information and warnings primarily from Prof. Büchi of the Anthropology Department. Details on the topic "university reform" also in Re 7), in re 14), and in SPECIAL PART A – text at the end of each of these paragraphs.
Responsible for this irresponsible law were the “Berliner Senator für Wissenschaft” Prof. W. Stein and G. Löffler, his successor.
[xii] See also the two “heptachlorodioxane” references from 2005 and 2006 in [19] and PROJ X in [1]
for correspondence: rriemschneider@yahoo.de
[ BWW Society Home Page ]
© 2009 The Bibliotheque: World Wide Society