|
Bonded to Compounds Providence
by Prof Dr Dr Randolph Riemschneider, L B Fel Life BWW Fellow Prof R Riemschneider, has summarized his life-time’s work in a soon to be published book (1) in which will be found a number of chemical compounds and natural products that, in a certain way, seem to have developed a life of their own. Editor
In Riemschneider's NACHLESE - 66 JAHRE CHEMIE (Re-reading 66 years of chemistry / 66 years of chemistry re-read) subdivided into XXVI projects (1), some compounds and natural products are conspicuous which have played a special role in the author's life over many years - one could almost say that they have "pursued" him to this day, to wit: a) o-diacetylbenzene (o-di) - from 1937 b) hydride ions - from 1940 (2) c) yeasts - from 1937 d) ATP - from 1941 e) aspirin - from 1941 f) thalidomide - from 1958 (3) g) heptachloro-1,4-dioxane(s) - from 1943 h) dichlorodiphenyltrichloromethylmethane - from 1942 i) hexachlorocyclopentadiene, cylopentadiene - from 1943 Reports on two of these research complexes [b) and f)] were published in this journal recently (2,3), and were followed by an article on "heptachlorodioxane(s)" dealing with a purely academic problem. Elucidations
for a) to i) follow: a) ortho-diacetylbenzene
(o-di) [in loc cit (1) PROJECT I]: This compound, when finely divided an inconspicuous
white powder that stains the skin, ie suited for marking, already came into
play during the author's school days (4). o-di and analogues [polyacetyl
chemistry developed by the auhor] have resulted in more than 100 publications,
lectures and patent applications. o-di can be used as an amino acid
reagent [ninhydrin substitute (5)], was tested and used as a marking agent by
the CID in instruction on
the market for many years. The numerous theses submitted for a degree or
doctorate in this field were scietifically very useful and also enjoyable for
the students, as eg the “telephone receiver affair” or “psoriasis treatment”
have shown: PROJECT I (1). b) Hydride ions [in loc cit (1) and (2)]: Since 1939, while studying chemistry, the
author concerned himself with hydride ions, to be more precise with the
questions of their existence in aqueous solutions, detecting them, their stability
and action as free-radical scavengers, whether they occur in mountain waters,
in water from c) Yeast [in loc cit (1) PROJECT XXII]: Like o-diacetylbenzene, yeast has also played a role in the author's life since his school days: 1937/38 "Studying the influence of baker's yeast on the potency of male mice" (9). Followed while studying chemistry by: A bibliographical paper on "Yeast and yeast extracts" in the colloquium of the Institute of Chemistry at the University of Göttingen (10) and a lecture on "Respiration and Fermentation" in the Organic Institute at the University of Hamburg (11), followed by practical experiments in the Institute of Hygiene at the University of Jena with the WARBURG-method: manometric measurements to determine the absorption or release of smallest quantities of the gases O2 or CO2 (12). Then continuing work on several yeast "topics" for over 40 years: PROJECT XXII. Via newly developed yeast cell preparations, the author came to the vegetal feed additive known as PROVAL or H 2000 (25, 26), as well as to cosmetic additives, called CYTOCATALYZER, a commercial preparation in Asia for 15 years. d) ATP [in loc cit (1) above all PROJECTS XXII and XXIII]: ATP (adenosine triphosphoric acid), isolated from muscle tissue and elucidated by Prof Dr K Lohmann in 1937, is a compound which plays a central role in the intermediary metabolism, to be precise as the "high-energy" reaction product of the most important exergonic reactions like respiration (biological oxidation) or glycolysis (in yeast: fermentation), localized in the mitochondria or cytoplasm of the cell. Mitochondria are also known as the "cell's power stations". The author first met Prof Dr K Lohmann, the
discoverer of ATP, in his capacity as the director of the Institute of
Physiological Chemistry at the Kaiser Wilhelm University of Berlin in 1946,
when submitting to him, on the recommendation of Prof Rienäcker (Rostock), his
professorial thesis on the "Constitution and Action of Insecticides",
that had been partially written in industry and at Jena University. After
careful perusal, Lohmann accepted the paper and offered the author a position
in his institute for the duration of the proceedings, and the opportunity to do
his own research, both as a research assistant and lecturer. Special
circumstances resulted in the author having to conduct the repeat intermediary
preclinical exams in physiological chemistry for medical students, meaning that
accordance with the regulations he had to go with the candidates to a full
professor, and so got to know the famous surgeon Prof Sauerbruch. After one
such exam he asked: "Why do wounds heal faster if one puts organ material
or muscle flesh on them?" The author instantly saw a possible link with
the ATP isolated by Lohmann from muscle tissue, and proposed the hypothesis
that “more proteins are formed through increased ATP production (energy
increase) and available for the healing process”. Following up the hypothesis
and putting it into action from 1947 to 1970 led to very important results in
the field of organ extracts and led, for example, to a remedy licenced in Japan
since 1972 as well as certain bases for assessing the quality of cosmetic
additives: measuring the increase in the skin's metabolic activity after
applying extracts of placenta, thymus and blood. CELLRYL, the medicine based on
calf blood extracts developed by the author for treating stomach ulcers, was in
use in e) Aspirin [in loc cit (1) PROJECT I] Aspirin is among the compounds that "had a decisive influence on the author's life". The substance "developed a sort of life of its own": at the request of Prof Dr C Weygand, head of a department in the Institute of Organic Chemistry at the University of Leipzig, the author gave two lectures[2] on aspirin in 1941 at the colloquia for deepening training in organic chemistry (13). The author learned a great deal about aspirin's properties and effects by studying the literature and in personal discussions with Weygand. Immediately after graduating as a chemist (diploma of 01 Oct 41), the author was, on 03 Oct 41, called up into the infantry, where he got to know a demoted police officer named Erdmann who became his teacher in matters of "how to get away from the infantry". Aspirin the author got from Erdmann helped here, namely 7 tablets to cause his heart and pulse to race. It worked, the captain in the medical corps transferred the author to the National Guard, in any event far away from the terrible infantry service and the attendant risks. The next help from aspirin came in autumn
1945, when the Soviet occupiers in Then in old
age, the author took a 100 mg "aspirin protect" every day for several
years in order to prevent infarct - but according to recent research aspirin
apparently merely stops inflammation. To stop atherothrombosis and so infarct
or stroke “iscover” for instance, based
on clopidogrel might be a viable alternative. Aspirin also played a role in the field of
research over the years: the author tried to undertake something against the
salicylic acid that forms in the stomach after the acetyl group splits off (13,
lecture III) and suggested developing an "apririn protekt"
(encapsulated) to Prof Dr Otto von Bayer, head of research at BAYER Werke, in
1953 - both in talks and in writing. But the cooperation envisaged did not
materialize. The author's suggestion and request to test the aspirin analogues
he to be synthesized by him - in which the carboxyl group is replaced by
another - for antiphlogistic action (14), was also not accepted. It was
realized from 1977 by experiments in f) Thalidomide [loc cit (1) PROJECT XXI and (3)] The author has been interested in thalidomide since 1958, ie immediately after it came on the market as a seditative, inasmuch as he saw in it an unphysiological amino acid derivative, derived from glutamic acid. The author had sought such compounds since 1947, in order to disrupt the metabolism of insects, ie to put a less dramatic process alongside “halo compound-based chemical pest control”. As the manufacturers of thalidomide did not see fit to make it available to the author, he synthesized it in his own lab [loc cit (3), cit 9, 16]. Deformities were observed during experiments in various directions with thalidomide on tadpoles [loc cit (3) cit 17, 18], as well as inhibition of ascites cells [loc cit (3), cit 3]. When he was in Thalidomide is licensed as a cancer
therapeutic agent in the g) Heptachloro-1,4-dioxane(s) A short report on this purely academic subject from the field of stereochemistry follows these explanations later in the text (45 years of research). h) Dichlorodiphenyltrichloromethylmethane [loc cit (1) in PROJECTS VI to VIII]: In 1942 fell Gesarol, a pest control agent against the potato beetle, into the author’s hands for analysis. It proved to be C14H9Cl5, synthesized from chloral and 2 mol chlorobenzene (Bayer condensation), become famous late in the second world war as DDT (16, 17). In 1942 experiments on flies with Gesarol showed that the agent only acted after some time by contact (flies are sucking insects and can not imbibe a practically water-insoluble compound like food). Under the title “Halo carbon-based contact insecticides” (18), the author synthesized approx 70 analogues (note: before the compound became famous as DDT) and several hundred other halo compounds that were systematically tested after the war, of course continuous orientation experiments with flies and bollweevils from 1942 (cit 16). DDT led the author systematically to new, more effective contact insecticides, as is shown in i). These investigations enabled the author to qualify early as a professor as both his doctoral and professorial theses were written simultaneously while working in petro-industry. In addition, he wrote two monographs about contact insecticides (19) in 1947 and 1949 that led to important international contacts. i) Hexachlorocyclopentadiene (C5Cl6), cyclopentadiene (C5H4), [loc cit (1) in PROJECT XI]: The author made C5Cl6 by the action of hypochlorite on C5H6 in the Explosives and Warfare Agents Establishment in Prague in order to study its characteristics and compare them with the explosive dichloroacetylene (C2Cl2) (20). Employed at RUHRÖL GmbH’s hydrogenation works from 01 Apr 43, the author pulled (in addition to his regular work) C5Cl6 as the starting material for the synthesis of “Halo compounds with new spatial distribution of chlorine” (21, 22) in the frame of the subject “Constitution (configuration) and activity of halocarbon-based contact insecticides”, cf h) too. This work led to the discovery of a new group of insecticides that was 1000 times more potent than DDT, namely M 410 (23, 24) – aka chlordan in America. Contrary to all expectations, C5Cl6 entered into a DIENE synthesis, in part under exothermic reaction. In collaboration with Farbwerke HOECHST this work later led to the insecticide and commercial product THIODAN: loc cit (1) PROJECT XI. The author sees
in what is described above a sort of natural act of fate, similar to the events
described below relating to “Prague” and “survival” – as if guided by an
invisible hand. This red thread of providence is still spun today. The author was evidently intended to go to When he was discharged from the military
hospital in The author was evidently intended to survive: Several times his life was in danger,
“escaped bombs”once in The author was awarded his doctorate in Thrice 22 Sept: On 22 Sept 42: army doctor in
Dniepropetrovsk diagnosed infectious jaundice: repatriated to On 22 Sept 43: explosion in the lab of the hydogenation works where the author was meanwhile employed, only 1st and 2nd degree burns in the face. On 22 Sept 44: in hospital in Bibliography
(1) R Riemschneider: “Re-reading 66 years of chemistry” with approx 1,500 citations (own publications, lectures, lab reports, patents) and descriptions of projects I - XXVI plus vita (in preparation). (2) R Riemschneider: Nutritional Supplement by Hydrid Ions acting as Antioxidants and Hydrid Ions and H Atoms as “Energy Currency” for Living Systems, 5 p. The Bi-Monthly Journal of the BBW Society, 2004 http://www.bwwsociety.org/journal/html/hydrid.htm (3) R Riemschneider: Thalidomide: A remedy with two faces, 15 p, The Bi-Monthly Journal of the BBW Society, 2004 http://www.bwwsociety.org/journal/html/thalidomide.htm (4) R Riemschneider: Oxidation of ethyl benzene, o-,m-,p-diethylbenzene nd o-ethyl acetophenone with potassium permanganate in buffered solution – o-diacetylbenzene, (Oxidation von Ethylbenzol, o-,m-,p- Diethylbenzol und o-Ethylacetophenon mit Kalium-permanganat in gepufferter Lösung – o-Diacetylbenzol) year’s course work in chemistry for graduating from college, 1939, 96 p; pulished in Gazz Chim Italiana 77, 607-611 (1947), submitted on 12 Sept 40. (5) R Riemschneider, C Weygand, M Somplatzki,
J Wierer o-diacetylbenzene
as amino acid reagent (o-Diacetylbenzol
als Aminosäure-Reagens) Lab reports 1947-51, 105 p; Mh Chem 86, 201-209 (1955); Z analyt Chemie 193, 186-189 (1962) (6) loc cit (1) in PROJECT I (74, 87) (7) R
Riemschneider, K Nolde, K Henning, Preparing
o-diacetylbenzene (Darstellung von
o-Diacetylbenzol)
Mh Chem 104, 987-989 (1973) o-di from 1,4 dimethylnaphthalene (o-Di aus 1,4-Dimethyl-naphthalin) Mh Chem 93, 616-617 (1962) (8) Norman
“Fresh
vegetable and fruit juices”, Norwalk Press, “Become younger”, “Strahlende Gesundheit” Mosaik, W
Goldmann-Verlag, Munich, (9) R Riemschneider, A Suhr, H Kahl On the negative influence
of normal, intact yeast (baker’s yeast) on the
potency of male mice (administered in drinking water) (Über den negativen Einfluß normaler
intakter Hefe (Bäckerhefe) auf die Potenz von Mäuseböcken (verabreicht im
Trinkwasser), Lab report, 15 p, June 38. Abridged version published in school magazine of Wilhelm Grammar School, Hamburg-Dammtor, 1938. Result: inhibition of potency approx 20%. (10) R Riemschneider, A Suhr, A Kersting “Yeast and yeast extracts – Saccharomyces cerevisiae HANSEN – biocomplex yeast” („Hefe und Hefe-Extrakte – Saccharomyces cerevisiae HANSEN – Biokomplex Hefe“) Lecture, given in chemical colloquium of Anat Chem Dept, Inst of Chem, Univ of Göttingen, mid-Dec 39 (11) R Riemschneider “Fermentation and
respiration” (Gärung und Atmung)
Part
of lecture given in colloquium of Inst of Org Chem, Univ of (12) R Riemschneider WARBURG
method – first practical experiments on “Fermentation and respiration” (WARBURG-Methodik – Erste praktische Versuche zum Thema „Gärung und Atmung) in Inst of Hygiene,
Univ of Jena, Lab reports, 17 p, Nov 44
(13) R Riemschneider
“o-acetylsalicyclic acid
(aspirin)”
(Acetylsalizylsäure (Aspirin): 3 lectures:
1 : “Acetylsalicylic acid and its relationships to
salicin [Salix Cortex salicis (willow
bark), methyl salicylate [gaultheria varieties wintergreen leaves) and
salicylaldehyde [Spiraeae (Meadowsweet Flower)] (Acetylsalizylsäure und ihre Beziehungen zum Salizin
[Salix Cortex Salicis (Weidenrinden)],
zum Methylsalizylat [Gaultheria-Arten (Wintergrünblätter)] und zum
Salizylaldehyd [Spiraeae
(Meadowsweet Flower)])
Lecture given in colloquium of Org
Chem Dept, Inst of Chem, Univ of Leipzig, May 41
2
: “On the history of aspirin[3] and its effects” (Zur
Geschichte des Aspirins und seine Wirkungen) Lecture given as lecture 1, June 41 3: “Aspirin: hypothesis on antiphlogistic action of aspirin” (Aspirin: Hypothese zur entzündungshemmenden Wirkung des Aspirins) Lecture given in colloquium of Inst of Hygiene, Univ of Jena, 15 Oct 44 Presentation of hypothesis in lecture 3 that aspirin’s antiphlogistic effect is due to it acetylizing certain NH or OH groups (serine, thereonine?) in the union of peptide chains of enzymes (E) (Eq 1). (Eq 1): Aspirin + E = E-COCH3 + salicylic acid Certain (eg gastrointestinal) side effects are caused by the formation of salicylic acid (Eq 1). To avoid forming salicylic acid, aspirin’s ortho position acid group had to be replaced by other substituents, naturally while maintaining the aspirin analogue’s antiphlogistic action. Another possibility was gone into at the end of the, namely enclosing aspirin in capsules that could pass through the stomach uneffected, ie would only dissolve in the intestines. (14) R Riemschneider, F R Pesserl, O Goehring, M M Faria, H Kahl Synthesizing
55 aspririn analogues as per 1944 hypothesis (Synthese von 55 Aspirin-Analogen, gemäß Hypothese aus dem
Jahr 1944)
Lect 3 (13). From: Inst of Biochem, FU Berlin, Central Inst of Chem; fed univ of S Maria (UFSM), Santa Maria, RS, Brazil; lab of Consulting, Development and Engineering, S Paulo and Rio de Janiero, Brazil. 8 lab reports from 19676-87, 121 p (15) M M Faria, R Riemschneider, F R Pesserl, H
Ferreira Cyclooxygenase-inactivation
by aspirin analogues (Cyclooxygenase-Inaktivierung
durch Aspirin-Analoge)
Lab reports 1987, institutions as above (14) (16) R Riemschneider Extracting active agent of Gesarol and identifying lipoid-soluble part as C14H9Cl5 (Extraktion des Wirkstoffes Gesarol und Identifizierung des lipoidlöslichen Anteils als C14H9Cl5 ) - with the aid of GEIGY patents reviewed in Chem Zentralblatt – more precisely as condensation product of chloral and chlorobenzene (17) Lab reports, 8 p, May 43 (17) Preparing b,b,b-trichloro-a,a-bis-[4-chlorophenyl]-ethane
(dichlorodi-phenyltrichloromethylethane) from 1 mol chloral and 2 mol
chlorobenzene as proof of structure of isolated Gesarol active agent (Herstellung von β,β,β-Trichlor-α,α-bis-[4-chlor-phenyl]-ethan
(Dichlordiphenyltrichlormethylmethan) aus 1 Mol Chloral und 2 Mol Chlorbenzol,
zum Konstitutionsbeweis des isolierten Gesarol-Wirkstoffes) Lab reports, 6 p, June 43. Experiments conducted in
works lab of RUHRÖL GmbH, (18) R Riemschneider Coining the term “halocarbon-based contact insecticides” (Prägung des Begriffes „Kontakt-Insektizide auf Halogenkohlenwasserstoffbasis) June 43 –planning, Lab reports 1943 (19) R Riemschneider Contact insecticides I and II (Zur Kenntnis der Kontakt-Insektizide I und II) 1st suppl vol, 2nd and 9th special issue of “Die Pharmazie” 1947 and 1949, pp 77-99 and 649-800 (20) R Riemschneider (lecturer), A Kühnl, O
Schmidt Hexachlorocylopentadiene, C5Cl6: 2 lectures 1: “The
action of sodium hypochlorite on cylopentadiene: hexachloropenta-diene and
higher-melting reaction products” (Über die Einwirkung von
Natriumhypochlorit auf Cyclopentadien: Hexachlorpentadien und höher schmelzende
Reaktionsprodukte) Lecture given on 15 Feb 43 (20 p) 2: “Properties and reactions of C5Cl6” (Eigenschaften
und Reaktionen des C5Cl6) Lecture given in German Army Explosives and Warfare
Agents Research Estab-lishment in The “example” of dichloroacetylene, C2Cl2, made it seem appropriate to test if C5Cl6 was also suited for an explosive or warfare agent. Result: negative (21) R Riemschneider Constitution and properties of wax-like
side products, and their properties, obtained when preparing
hexachlorocyclopentadiene from cyclopentadiene and hypochlorite. Possible new
DIELS-ADLER adducts (Konstitution und Eigenschaften
der bei der Herstellung von Hexachlorcyclopentadien aus Cyclopentadien und
Hypochlorit entstehenden wachsartigen Nebenprodukte und deren Eigenschaften.
Mögliche neue DIELS-ALDER-Addukte,) Lab reports, 30 p, RUHRÖL GmbH, Bottrop, Feb 44 (22) R Riemschneider On using
hexachlorocyclopentadiene as starting material for “synthesis of chlorine
compounds with new spatial distribution of chlorine”: OET group (later: DIENE
group) (Über den Einsatz von Hexachlorcyclopentadien als Ausgangsmaterial zur
„Synthese von Chlorverbindungen mit neuer Verteilung von Chlor im Raum“: OET-Gruppe
(später DIEN-Gruppe)) Lab reports, 9
p, Dec 44. Experiments conducted at RUHRÖL GmbH, (23) R Riemschneider “A new type of insecticidal halogen compounds,
starting from C5Cl6 – after first tests, very promising:
M 410” (Ein neuer Typ insektizider
Halogenverbindungen, ausgehend von C5Cl6 - nach ersten Testversuchen vielversprechend:
M 410.) Lecture given in Inst of Pharmaceutics, Univ of Jena on 31 Jan 45 Insecticidal activity of M 410 on Calandra granaria & Blatta orientalis greater than DDT (24) R Riemschneider On the
remarkable properties of post-chlorinated adducts from
hexachloro-cyclopentadiene and cyclopentadiene, C10H6Cl6
– M 410 identified as octa-chloroendomethylenetetrahydrohydrindene (OET),
C10H6Cl8 (mol wt 410) (Über die bemerkenswerten Eigenschaften
nachchlorierter Addukte aus Hexachlorcyclopentadien und Cyclopentadien, C10H6Cl6.
– M 410 identifiziert als Oktachlorendomethylen-tetrahydrohydrinden (OET), C10H6Cl8
(Molgewicht 410))
Lab reports 1944 and to Mar 45. Experiments conducted
at RUHRÖL GmbH and in Inst of Hyg, Univ of Jena (25) R Riemschneider Cytocatalyzer, Seryel, and
Collaplant http://www.bwwsociety.org/journal/html/cytocytalyzer.htm (26) R Riemschneider Two Notes on Progress in BSE-Crisis
(H 2000), 2002 http://www.bwwsociety.org/journal/html/bsicrisis.htm About so-called
heptachloro-1,4-dioxane from m p 122oC by and Central Santa Maria, Rio Grande do Sul,
Brazil Abstract: For studying the problem of chair-conversion-isomery during 1947-1986 (4) we prepared many 1,4-dioxan chlorination products and analysed them for the two theoretically possible heptachloro-1,4-dioxane (I)-isomers. Of the two described I-isomeres, melting at 57° C and at 122° C, only the first does actually exist. Keywords: dioxane – heptachloro-1,4-dioxanes (I) – chair-conversion-isomery We researched
the chlorination of 1,4-dioxane and some of its crystallized Cl-substitution
products intensively at the FU Berlin and in various institutions in Brazil
from 1947 to 1986, and we were able
to isolate only one heptachloro-1,4-(I)-isomer from all the final
chlorinated products, namely the one that melts at 56-57oC – unlike
H Stumpf (3) who some 50 years ago described a second I-isomer from m p 122oC.
Two I-isomers would have been interesting inasmuch as we would have had two
partners in a conversion relationship to one another seperate in substance for
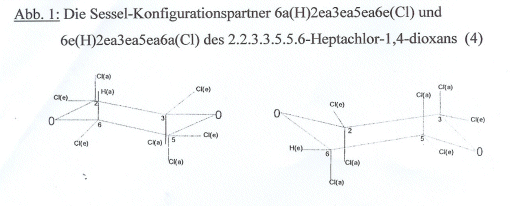
the first time (4): Diag 1 Diag 1: The chair configurational
partners 6a(H)2ea3ea5ea6e(Cl) and 6e(H)2ea3ea5ea6a(Cl) of
2.2.3.3.5.5.6-heptachloro-1,4-dioxane [lit (4)] Key to diag 1:
In an imagined conversion of the 1,4-dioxane chair, in which eg the C atoms 2
and 6 and the O atom 4 migrate down and the C atoms 3 and 5 and the O atom 1
up, 2 chair configurations of 1,4-dioxane substitution products pass over into
one another, that we combine to chair configuration pairs. Of the partners in
such a configuration pair, we call the original chair configuration “body” and
the converted one “counter body”. In the case of
2.2.3.3.5.5.6-heptachloro-1,4-dioxane, the body has the configuration
6a(H)2ea3ea5ea6e(Cl) and the counter body 6e(H)2ae3ae6a(Cl), ie on conversion
all e-connected substituents convert to a-connected and vice versa (e as in
equatorial, a as in axial). In the case of
monofluoro-enkekachlorocyclohexane, the body has the chair configuration
1e(F)1a2ea3ea4ea5ea6ea(Cl) and the counterbody 1a(F)1e2ae3ae4ae5ae6ae(Cl), both
realized (5,6). We sought
representatives, above all among high-substituted polyheterohalo-cyclohexanes,
which were in a conversion relationship to one another and could be synthesized
separated in substance; we succeeded in this at the time in the case of the two
monofluoro-endekachloro-cyclohexanes in collaboration with Y Morino,
Tokio and W Plieth, The author
already cast doubt on the existence of the “I-isomer from m p 122oC”
described by W Stumpf at the Chemists Congress in Innsbruck on 01 Apr 53
immediately after Stumpf’s lecture, saying it was improbable that a
heptachloro-dioxane would melt higher than the symmetrically formed
octachloro-1,4-dioxane (II), for which the author had determined a melting
point of 110-112oC and a dipole moment of 0.2 D (7, 9) in 1948. At the time References: (1) From
lecture and publication Special structure of heptachloro-1,4-dioxanes and
related compounds. Cit (677) in: R Riemschneider “Re-reading 66 years of
chemistry” (in preparation) (2) Loc
cit (1) cit (656-659), (662-667), (669-674), (677 and 678). Authors: R
Riemschneider with (in chronological order): K.Schürken, K.Lohmann, W.Gerischer, W.Cohnen, A.Heymons, F.Scherer, G.Seeliger,
R.Wasicky, W.Stuck, E.B.Grabitz,
D.Takei, P.Nowack, H.Fereira ,M
Azhar (1947-1986) und M.M.Faria, H.S.Chang, Chemical-Engineering-Development
(1977 –1982); cf (8) (3) W Stumpf Chemistry and applications of 1,4-dioxane (Chemie und Anwendugen des 1,4-Dioxans) Verlag Chemie GmbH 1956, p 130-133 (4) R Riemschneider About the configurations of 1,4-dioxane substitution
products (Über Konfigurationen von
1,4-Dioxan-Substitutionsprodukten) Z Naturforschg 8B, 745-751 (1954), Free (5) Y.Morino,
R.Riemschneider, (lecturer) M.Z. Azhar, W. Plieth: X-ray of 2 C6FCl11 – isomers,
report, 1986, 9 pp; Lecture from May 15, 1986, given in the Chemical Institute of SAITAMA University, (6) R Riemschneider, W Pollack,
F R Pesserl et al (1969-76) a) Long-term chlorination experiments to
isolate monofluoroendekachloro-hexane(s), starting from fluorobenzene,
difluorobenzenes, monofluorotri-chlorobenzene, pentachloromonofluorobenzene,
monofluoroheptachloro-cyclohexane from m p 218oC, from
monofluorohexachlorocyclohexane oils and many other fluorine derivatives:
(Langzeit-Chlorierungsversuche zur Isolierung von
Monofluor-endekachlorcyclohexan(en), ausgehend von Fluorbenzol,
Difluorbenzolen, Monofluortrichlorbenzol, Penta-chlormonofluorbenzol,
Monofluorheptachlorcyclohexan vom Schmp. 218°C, von Monofluorhexachlorcyclohexan-Ölen
und vielen anderen Fluorderivaten) PROJECT II 6 (1) b) Negative
results with respect to the further search for a second
hepta-chloro-1,4-dioxane isomer in chlorinated products of tetra- and
hexachloro-1,4-dioxanes. (Negative
Ergebnisse hinsichtlich der weiteren Suche nach einem zweiten
Heptachlor-1,4-dioxan-Isomeren in Chlorierungsprodukten von Tetrachlor- und
Hexachlor-1,4-dioxanen) Mss, 12 p, 1976. Experiments from the Central Chem
Inst, Univ Fed of Santa Maria (UFSM), S Maria, RG, Brazil. The long-term chlorinations were conducted in Carius
(bomb) tubes that were exposed to sunlight on the institute roof for up to two
years. Of 300 tubes some 180 were lost through explosion.Such experiments were
no longer possible in (7) F Scherer, R Riemschneider
(discussant), R Wasicky Critical comments during
discussion of lecture by W Stumpf on “Dioxane-chlorinated products as
insecticides” (Kritische
Diskussionsbemerkung zum Vortrag von W. Stumpf über „Dioxan- Chorierungsprodukte als Insektizide) Chemists Congress, Text of comments sent to Österreichische
Chemiker-Zeitung, (8) Loc cit (2) eg a) R Riemschneider, A Heymons, G Seeliger, E B
Grabitz (1954-56) Experimental testing of question of existence of second “heptachloro-1,4-dioxane” (m p 122oC) (Experimentelle Prüfung der Frage der Existenz des zweiten „Heptachlor-1,4-dioxans“ (Schmp. 122°C)) Ms 10 p, 1956. Unpublished experiments in Dept of
Biochem, FU Berlin and G Seeliger’s 30-page degree thesis, Tech Univ of Berlin,
1956 Despite the most intensive efforts, it did not prove
possible to isolate a second heptachloro-1,4-dioxane isomer by the time the
manuscript was written, nor in-deed later. b)
R Riemschneider (lecturer), E B Grabitz, J Takei, P Nowack (1958-62) zwei 2,2,3,3,5,5,6-Heptachlor-1,4-dioxan-Isomeren in
1,4- Dioxan-Chlorierungsprodukten
(ausgehend von 1,4-Dioxan sowie definierten Tetra- und
Hexachlor-1,4-dioxanen und vom Dioxadien). Lecture in
colloquium of Dept of Biochem, FU Berlin on 20 Jan 61 Lab reports from 1958-62 from above dept. Result up to
1986: negatice. There is only one
heptachlorodioxane isomer (680) (c) R Riemschneider, M M Faria, H Continuing 1,4-dioxane chlorination experiments. The heptachloro-1,4-dioxane problem (Fortsetzung der 1,4-Dioxan-Chlorierungsversuche. Das Heptachlor-1,4-dioxan-Problem) (commissioned research) 24-page report, 1983, of experiments conducted by
Chemical Engineering-Development of S Paulo, The non-existence of so-called heptachlorodioxane from
m p 122oC was also confirmed by this independent institution. (9) R Riemschneider, K Lohmann, W Gerischer,
W Cohnen, A Heymons (1947-49) About
preparing and identifying 10 Cl-substituted 1,4-dioxanes, dioxens and
subsequent products as well as testing them for contact-insecticidal activity
(direct chlorinations of 1,4-dioxane and 1,4-dioxane chlorinated products); 2,2,3,3,5,5,6-heptachloro-1,4-dioxane from m p
56-57oC and octachloro-1,4-dioxane from m p 110-112oC (Über die Herstellung und Identifizierung von 10
Cl-substituierten 1,4-Dioxanen, -Dioxenenund Folgeprodukten sowie ihre Prüfung auf
kontakt-insektizide Wirksamkeit (direkte Chlorierungen von 1,4-Dioxan und
1,4-Dioxan-Chlorierungsprodunkten); 2,2,3,3,5,5,6-Heptachlor-1,4-dioxan vom Schmp. 56 –
57°C, Oktachlor-1,4-dioxan vom Schmp. 110 – 112°.) Mitt
Physiolog Chem Inst Berlin, 15 p, Jan 49, secreted at the instigation of Dr F
Scherer, Farbwerke Hoechst AG; cf loc cit (1): (553); cf lect of Apr and May 52
in Berlin and Vienna loc cit (1): (446) [1] later bought up by MERCK [2] a third lecture followed in 1944 (13) [3] Aspirin was brought to medicine by BAYER in 1899. “A” from acetyl and “spir” from spiraeic (later: salicylic) acid
[ BWW Society Home Page ] © 2005 The BWW Society/The Institute for the Advancement of Positive Global Solutions |