Medicine & Psychiatry:
Excess of
Astrocyte Receptors in Tripartite Synapses May Cause a Severe Disorder of
Information Processing and Behavior in Depression
By Dr. Bernhard J. Mitterauer
Institute of Forensic Neuropsychiatry and Gotthard Günther Archives
Abstract: A biocybernetic model of the etiopathology of depression is proposed. It is hypothesized that an excess of astrocyte receptors in tripartite synapses may cause a significant delay of synaptic information processing. Therefore, the behavior generating systems in the brainstem reticular formation cannot decide in real time which mode of behavior is appropriate to a specific sensory information from the environment. The modes of behavior are described as all common psychobiological action patterns of human beings occurring in circadian time periods. A delay of synaptic information processing may lead to a displacement of the modes of behavior in the sense of a persistence of some modes (“must do”) and the incapability to produce others (“cannot do”). Such a severe behavioral disorder also affects the self-understanding of the patient resulting in a depressed mood. In a computer simulation of the decision processes in the brainstem reticular formation, it can be demonstrated that synaptic information processing delays lead to a displacement of the modes of behavior in depression. Preliminary clinical data support this model of depression. Finally, some consequences for the classification and biological treatment of depression are outlined.
Key words: tripartite synapses, astrocyte receptors, excess, information processing delay, reticular formation, modes of behavior, displacement, depression
Introduction
Depression is an extremely common disorder, ranking second in the global burden of disease (Murray and Lopez 1997). The core symptoms of depression are depressed mood, diminished interest or pleasure, disturbances of circadian rhythms, psychomotor disturbances (retardation or agitation) and feelings of insufficiency (worthlessness, etc.) (American Psychiatric Association 1998). In most patients, depressive episodes arise from the combination of familial, biological, psychological and social factors, operating over time and progressively increasing the risk of developing a depressive disorder (Akiskal 1995).
Here, I propose a novel biocybernetic model that may be explanatory for depressive behavior. My hypothesis is that if the information processing in tripartite synapses is severely delayed, the behavior generating systems in the brain stem reticular formation cannot decide in real time which mode of behavior is appropriate to a specific sensory information of the environment. This pathophysiological mechanism leads to a persistence of some modes of behavior (e.g. sleeping, eating, working, etc.) and, in parallel, the system is incapable of producing an appropriate behavior to changing situations in the environment. Such a severe disorder of behavior may essentially be caused by an excess of astrocyte receptors which cannot be sufficiently occupied by the cognate neurotransmitters, and thus, the information processing in tripartite synapses is protracted. Since our self-understanding is basically dependent on an effective behavior in the inner and outer environment, self-understanding is also affected in depression.
Before presenting my biocybernetic model of depression, the normal functions of information processing in tripartite synapses and the undisturbed generation of the modes of behavior in the reticular formation of the brain stem must be described.
Tripartite Synapses
According to the prevailing view, chemical synaptic transmission exclusively involves bipartite synapses consisting of presynaptic and postsynaptic components and a synaptic cleft, in which a presynaptically released neurotransmitter binds to cognate receptors in the postsynaptic cell. However, there is a new wave of information suggesting that glia, especially astrocytes, are intimately involved in the active control of neuronal activity and synaptic information transmission. Already in 1999, Haydon and coworkers showed that glia respond to neuronal activity with an elevation of their internal Ca 2+ concentration which triggers the release of chemical transmitters from glia themselves and, in turn, causes feedback regulation of neuronal activity and synaptic strength (Araque and others 1999). Accordingly, the signaling pathway at a tripartite synapse can be described as follows:
During synaptic neurotransmission, neurons release neurotransmitters from synaptic nerve terminals into the synaptic cleft to communicate with other neurons. The transmitter released from the synapse (or other co-released neurotransmitter) can - under certain circumstances - spill over from the synaptic cleft and reach neurotransmitter receptors in adjacent glial cells (astrocytes or perisynaptic Schwann cells), eliciting intracellular increases in Ca 2+ concentration in the glial cells (astrocytes). The increase in the astrocyte Ca 2+ concentration causes it to release a chemical transmitter from the astrocyte (“gliotransmitter”) that feeds back to the presynaptic nerve terminal to modulate synaptic neurotransmission (Fig. 1).
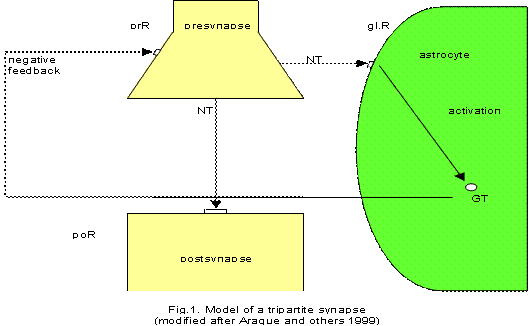
This figure is very schematic, showing only the basic signaling pathways at a tripartite synapse. Neurotransmitters (NT) are released from the presynapse occupying postsynaptic receptors (poR). In parallel, NT occupy glial receptors on the astrocyte (glR) activating Ca 2+ waves. The increase of Ca 2+ in the astrocyte causes it to release gliotransmitters (GT). GT feed back negatively to cognate presynaptic receptors (prR), so that neurotransmission is temporarily turned off. (Modified after Araque and others 1999).
Meanwhile, various models of a tripartite synapse are proposed (Auld and Robitaille 2003). In particular, the role of the glutamatergic tripartite synapse has been documented over the last years. The astrocytes produce glutamate as a transmitter by which they negatively feedback on the presynaptic element of the synapse. In other words: in tripartite synapses glia have a temporal boundary-setting function in temporarily turning off synaptic information transmission (Mitterauer 1998). What my model of depression concerns, I will especially focus on the role of such astrocyte receptors in synaptic information processing that have already been identified for various neurotransmitters (Haydon and Araque 2002).
Generation of the modes of behavior in the
reticular formation
According to Iberall and McCulloch (1969), a living system like man is highly dynamic. In order to produce an integrated behavior, it must be capable of generating stable system states, the so-called modes of behavior. We do not normally think of human behavior as modal, though most people would agree that their quality of consciousness is unitary and that they can only do one thing well at a time (Kilmer and others 1969). This may be identified as a dynamic action mode of the system, such as “the system sleeps”. In Table 1, the essential modes of behavior or action modes are listed which will have a time constant of the order of a female menstrual period. Although the list itself could be questioned, we would like to focus on the explanatory power of this scientific approach.
Table 1. Frequencies of the modes of behavior within about a 4
week cycle (Iberall & McCulloch 1969)
Modes of behavior Percent of time
Sleep 30
Eat 5
Drink 1
Vomit 1
Sexual activity 3
Work 25
Rest (no motor activity, indifferent 3
internal sensory flux)
Talk 5
Attend (indifferent motor activity, 4
involved sensory activity)
Motoric activity (run, walk, play, etc.) 4
Feel angry 1
Escape (negligible motor and sensory 1
input)
“Anxious-es” 2
“Euphorics” 2
Laugh 1
Aggress 1
Fear, fight, flight 1
Interpersonal communication (verbal or 8
sensory contact)
Envy 1
Greed 1
Total 100 ± 20% of
Time involvement
___________________________________________________________________________
McCulloch (1966) has associated the ability of the brain to integrate its function with the reticular formation in the brainstem, in the sense of an “integrative matrix” (Hobson and Scheibel 1980) based on the principle of redundancy of potential command. Over time, however, the reticular formation seems to have attracted the interest of scientists in its role as an activating or arousal system (Steriade 1996). Since the 1980s, we have further elaborated McCulloch’s theory of the RF (Mitterauer 1983; 1988; Mitterauer and Kopp 2003). Recently, a graph-theoretic model of the reticular formation has been proposed, but not referring to the principle of redundancy of potential command (Humphries and others 2006). The principle of redundancy of potential command means that the neurons or modules with the most information have the most authority. For a better understanding of this principle, McCulloch (1965) gave the example of a World War I naval fleet, where the behavior of the whole fleet is temporarily controlled by the signals from whichever ship first sights the enemy. The point is that this ship need not be the flagship in which command normally resides.
To realize the principle of redundancy of potential command, the reticular formation operates by an abductive logic (abduction). Abduction dates back to Aristotel. Peirce (1931-35) sees abduction as a type of inference yielding an explanatory hypothesis, rather than a result of deductive application of a ”rule” to a “case” or establishment of a rule by induction. In a more technical language, abduction is the selection of the appropriate program from a repertoire in accordance with a rule for analyzing program requests. These programs are general in the sense that all are principally adapted for the processing of environmental information. However, at the same time they are highly specialized in processing specific environmental information. When specific environmental information acts on the system, the system can decide or select to which program this information belongs, i.e. which program is best suited for information processing. The repertoire of these programs represents a heterarchic system (circular system) equipped with a redundancy of potential command. So, the reticular formation “starts out with rules: from this you run away: that you eat, etc. It starts out with these rules; it is presented with the fact, and it makes a guess that that fact is a case under that rule. This is the diagnostic procedure, the abductive procedure” (McCulloch 1966).
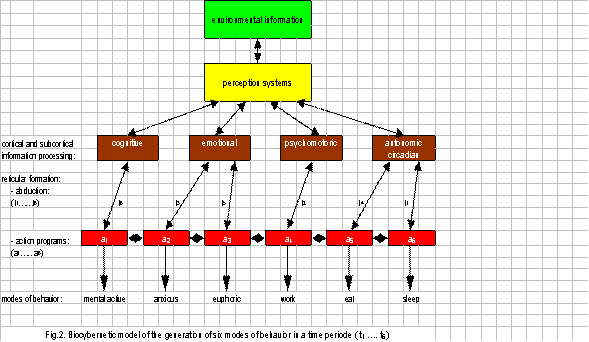
Figure 2 depicts a biocybernetic model of the generation of six modes of behavior in a time period (t1…t6). Environmental information from the perception systems is processed in specialized cortical and subcortical systems. For the sake of simplicity, it is only referred to the cognitive, emotional, psychomotor and autonomic-circadian systems. At the moments t1 to t6 the decision systems in the reticular formation decide based on abduction which information quality is most appropriate to an action program (a1…a6). Dependent on the action program activated, the integrative function of the reticular formation is capable of commanding and controlling the generation of a pertinent mode of behavior, such as mental activity, feelings of anxiety or euphoria, or to work, eat and sleep.
In addition, the cortical and subcortical information processing systems are interconnected (double headed) with the perception systems selecting environmental information. From a pure biological point of view, what is relevant for an intended task may be dependent on the extensive communication network that links the prefrontal cortex with other areas that process information arriving from senses or gained through experience and stored in memory. So the prefrontal cortex is connected in a topographically specific manner with most other cortices, including sensory and high-order association areas and subcortical structures associated with cognition, memory and emotions (Barbas and Zikopoulos 2007). Here, I will concentrate on the delayed synaptic information processing in these systems that may be essentially responsible for depressive behavior.
Delayed information processing in
tripartite synapses
As already hypothesized, if the receptors on astrocytes in tripartite synapses are increased such that a relative lack of neurotransmitters arises, the information processing will be delayed. As Figure 3 illustrates, the overproduction or excess of glial receptors (gl.R) leads to a prolonged production of gliotransmitters (GT) (dashed line) and, therefore, the negative feedback mechanism is protracted. The excess of glial receptors may be caused by mutations in those genes responsible for the expression of glial receptors for the various neurotransmitter types activated spontaneously or exogenously (stress, etc.). Originally, I assumed that the overexpression of glial binding proteins (Smit and others 2001) could be responsible for the delay of synaptic information processing (Mitterauer 2004). Unfortunately, these proteins have so far only been identified in molluscs.
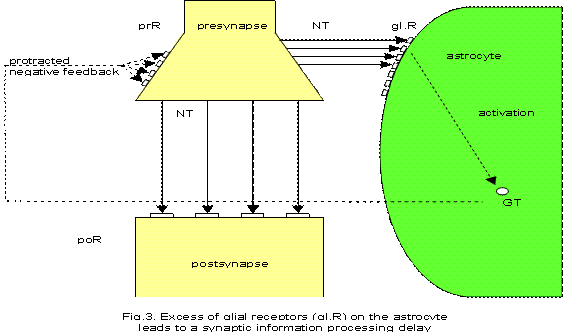
One may argue that brain diseases such as Parkinson’s disease also show a lack of neurotransmitter in pertinent synapses, but this is not necessarily accompanied by a depressive mood. The frequency of depression in Parkinson’s disease is estimated to lie between 30 and 40% (Poewe 2007). Hence, depression may occur only if the glial system is also affected.
From a psychological point of view, patients with depression have high aspirations (Bibring 1953), but these are unfeasible. One can also say that these patients are hyperintentional. Therefore, the excess of glial receptors on astrocytes may represent a biological explanation for both the high aspirations and their unfeasibility, since in such synaptic system states there are not enough neurotransmitters available for occupancy of all glial receptors. Thus, the mood is depressed. In addition, this pathophysiology of delayed synaptic information processing could explain symptoms of depressive retardation of thinking, concentration and motor behavior. Most importantly, a delay of synaptic information processing may also affect the generation of the modes of behavior in the brainstem reticular formation in the sense of a displacement of the behavioral pattern as typical of depression.
Displacement of the modes of behavior in
depression
In an environment that is temporal as well as spatial in nature, the nervous system needs to deal with various forms of internal or external delay. Internal delay can cause serious problems because by the time the central nervous system receives an input from the periphery, the environmental state is already updated (Lim and Choe 2006). In depression, an excess of astrocytic receptors in tripartite synapses may cause a severe delay of information processing leading to a displacement of the generation of the modes of behavior. In a computer simulation we are able to show how such a delay of synaptic information processing displaces the pattern of the modes of behavior. The computer system applied processes the information from the sensory systems or synapses and selects the appropriate pattern of modes of behavior according to the principle of redundancy of potential command (for technical details see Zinterhof 2006). Basically, the integrative systems in the brainstem reticular formation must rapidly decide which mode of behavior is most appropriate to a given situation in the environment. If this is not the case, the self-maintenance of the whole system is endangered. Our model and computer simulation of the decision processes in the reticular formation is not based on non-linear brain dynamics and does therefore not operate stochastically. Bearing in mind that decision processes in the brain are constrained by a myriad of hidden elements, the theory of non-linear dynamics defining decision processes as phase transitions (Werner 2007) could also be an explanatory approach for the displacement of behavior in depression. In contrast, how such a disorder may arise according to our model can be demonstrated by a simulation example.
Simulation
example of a hypersomnic depression
In our standard model of the implementation of the principle of redundancy of potential command, all subsystems (neurons, synapses, receptors) operate synchronously. This organization secures a real time information processing of the synaptic input from the sensory systems and also correct decision making in the reticular formation based on this incoming information.
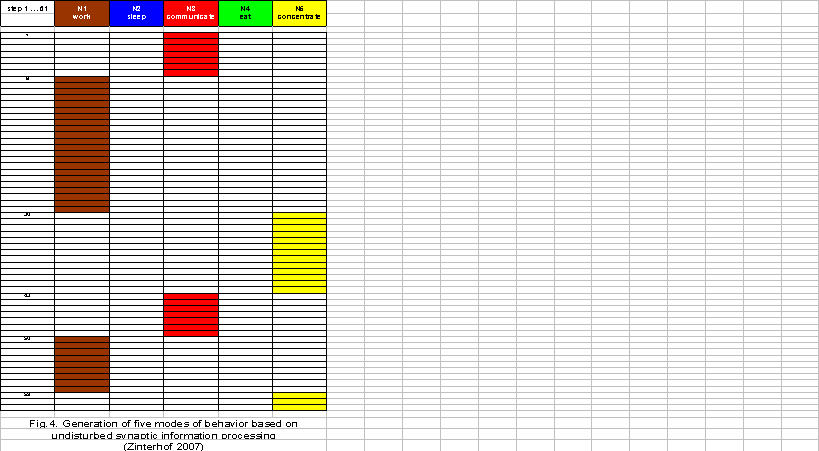
Figure 4 represents 61 steps of the simulation by selecting five modes of behavior. Taken the behavior of a housewife in the late morning as an example, first she communicates (N3), then she prepares a meal (N1) and concentrates on an interesting radio report (N5). After a short communication during a phone call (N3), she continues her house work and again she concentrates on the radio report (N5). The modes of sleeping and eating do no occur in the sense of a normal behavior period in the late morning.
Now supposing a temporal delay of information processing in tripartite synapses in line with the model proposed here, the system will continue to operate on the principle of potential command, but in a modified manner. The point is that the update of the neuronal network in the reticular formation operates at a higher frequency than the synapses of the sensory information processing systems. The intensity of the displacement of the modes of behavior may depend on the duration of phases in which the synapses do not transfer information to the decision network in the reticular formation. The behavior displacement shown in Figure 5 represents a proportion of one to two with concern to the neuronal updates in the networks of the brain stem and the synaptic updates of the perception systems. Here, a significant period of sleeping with a less frequent change to other modes of behavior takes place. The housewife falls asleep already in the late morning. She is only able to eat and shortly communicate on the phone. Most importantly, she is unable to keep up with her housework.
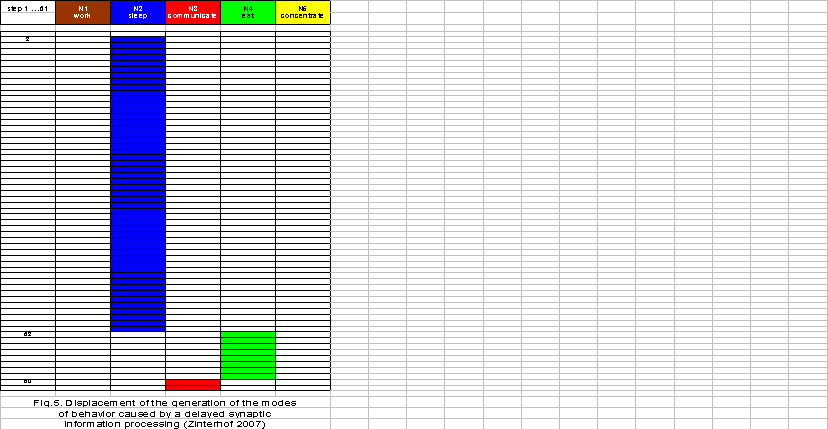
If the delay of synaptic information processing is further enhanced because of an increasing excess of astrocyte receptors, the neuronal network in the brainstem reticular formation needs more time (three time steps) to generate any mode of behavior at all. In addition, the generated mode of sleeping persists during the whole simulation period of 61 steps and does not change to any other mode of behavior (Figure 6). The housewife falls asleep during the late morning and is unable to work, communicate, eat or concentrate. Such a severe disorder of the usual behavior pattern of a housewife in the late morning hours can be diagnosed as a hypersomnic depression.
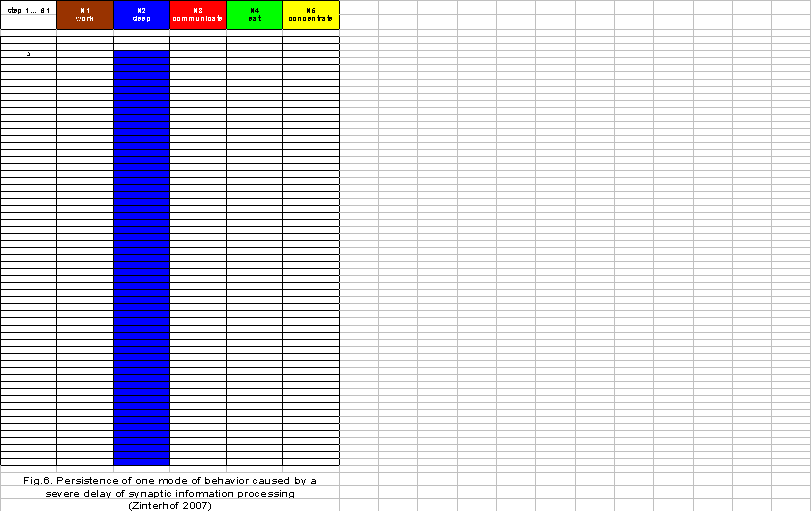
Depending on the degree of excess of astrocyte receptors in tripartite synapses and the brain areas affected, the severity of depressive symptoms will vary accordingly. For instance, if the prefrontal cortex is predominantly affected, a cognitive impairment will dominate the depressive symptomatology. The same holds for a prevailing delay of information processing in the limbic areas of the brain. The compulsive persistence of a mode of behavior is mostly not related to reality so that the capability to decide in real time which mode of behavior is appropriate to a given environmental information is at least temporarily lost. The patient cannot explain this severe behavioral disorder to himself, and therefore, he loses his self-understanding and a depressive mood is the consequence (Mitterauer 1994).
Preliminary clinical results
My novel model of depression has been tested on 30 patient with a major depression (DSM-IV 296) matched with a control group. The list of modes of behavior described by Iberall and McCulloch (Table 1) was elaborated to common human psychobiological behavior and a pertinent questionnaire (Salzburg Subjective Behavioral Analysis) was constructed for testing this model of depression (Rothuber and others 2007). Patients and controls were asked if the frequency of the 35 modes of behavior had changed during the last two weeks in comparison to normal, i.e. to a time of subjective well-being. To each question there is a choice of five possible answers, ranging from unchanged, seldom, more often to the extreme positions temporarily never or always.
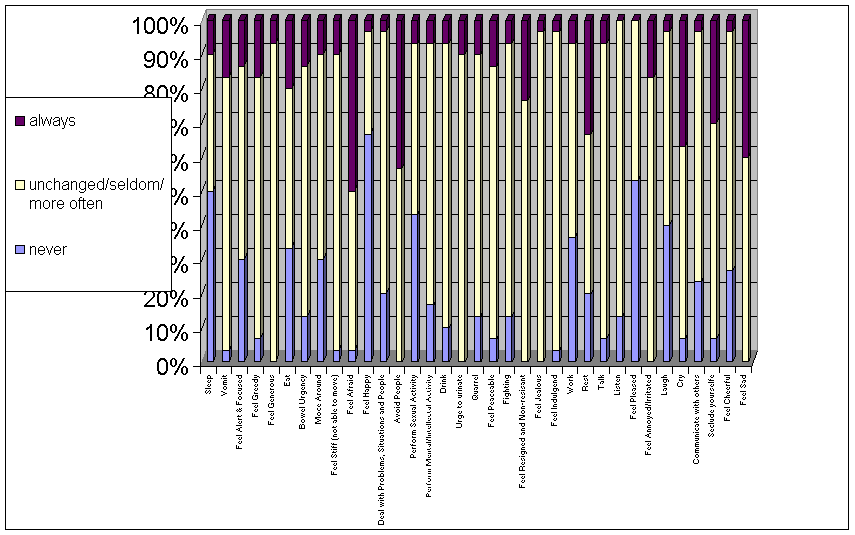
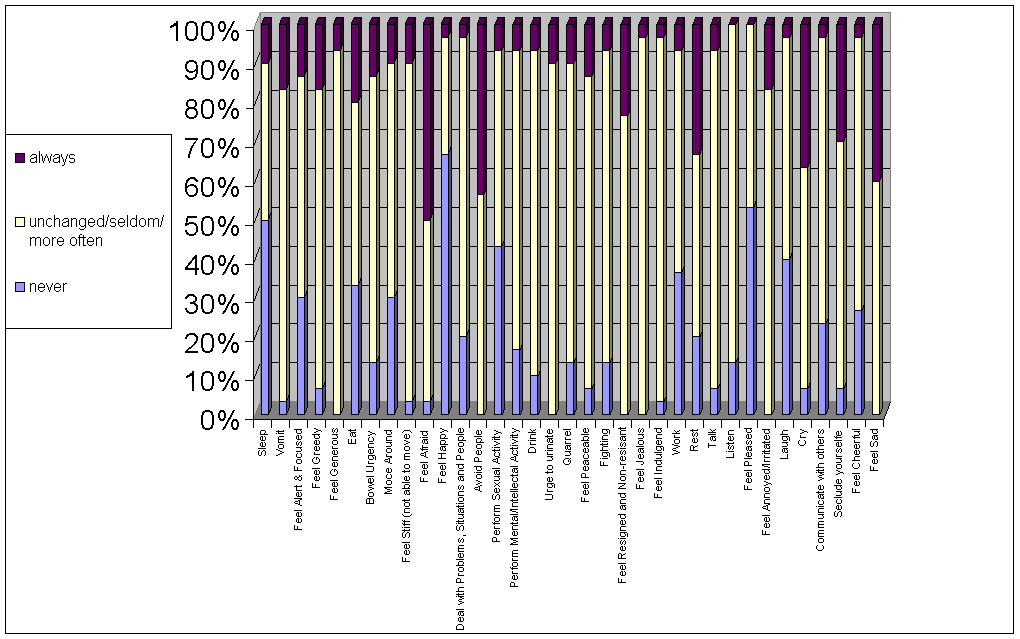
In
Figure 7 the extreme positions (always, never) are listed. Extreme positions
are reported from all 30 patients compared to only 2 of the healthy 30 control
subjects. A significant correlation was found between the frequency of extreme
positions and scores on the Hamilton Depression Scale (Spearman coefficient
r=0.44, p=0.02). However, the correlation between the
Krains (1957) describes in a seminal book so called atypical depressive forms characterized by a seemingly contradictory behavior with respect to the concept of depressive mood. So he speaks of a “smiling depression”. In our investigation, one severely depressed patient also reported and actually showed a persistent smiling. Whereas Krains attempts to explain such atypical depressions with psychodynamic arguments, my explanatory model is based on biological or biocybernetic mechanisms.
Future prospects
Current biologically based research on depression neglects the existential behavior generating and decision making networks in the brain stem, especially what the reticular formation concerns. Recently, two papers have been published that refer to the role of the brainstem in depression. Williams and Gordon (2007) suggest a frequent comorbidity of anxiety and depression. These authors suppose that in depression the accumulated effects of stress may disrupt hippocampal context-processing networks and lead to hypoarousal. However, a hypoarousal in depression could be an after-effect of a severe displacement or persistence of modes of behavior. The second study reports a reduced echogenicity in the raphe of the brainstem in depression (Walter and others 2007). It is speculated that this experimental finding may characterize a subtype of depression. However, both papers do not propose a theoretical conception of the reticular formation that could explain their experimental findings.
Considering my model of depression proposed, the possible cause of the delay of synaptic information processing is experimentally testable. At least the astrocyte receptors of neurotransmitters that play a role in the treatment of depression are already identified (Haydon and Araque 2002). By investigating post-mortem brains it can be tested if the astrocytic receptors in depression really outnumber those in normal brains. In vivo investigations with functional imaging techniques are also possible (Rothman and others 2002). In the case of an experimental verification of my synaptic model of depression, the degree of the excess of astrocyte receptors could be of special interest. First of all, a certain excess of astrocyte receptors may be occupied by an increase of neurotransmitter in the synaptic cleft caused by a reuptake inhibiting substance of neurotransmitters. However, in therapy resistant depressions the excess of astrocyte receptors may be in a range such that the therapeutic increase of neurotransmitter is insufficient. Here, a possible treatment procedure could be to block the excess of astrocyte receptors directly with a cognate substance.
From a clinical point of view, my model of depression allows a broader diagnostic approach and even a new phenomenological typology of depressive behavior. Let us take a closer look at the persistent modes of behavior. There are many psycholobiogical disorders with a phenomenology that is comparable to a persistent mode of behavior in depression. An eating disorder with vomiting behavior called bulimia is an impressive example as are non-substance addictions such as workaholics. Furthermore, so-called psychosomatic disorders as a permanent urge to urinate or defecate with a negative physical examination may be basically depressive symptoms. Interestingly, such disorders often show an improvement upon treatment with antidepressants. Here we may deal with masked depressions with underlying pathophysiological mechanisms of synaptic information processing and the generation of behavior.
If the behavior of a depressed patient is dominated by the persistence of one specific mode of behavior, the depression could be characterized by that mode. Taken the 35 modes of behavior listed in Figure 7, one can interpret each mode as a distinct phenomenological type of depression. Admittedly, some types may sound rather strange, as in “talking depression”. However, such a broad classification only makes sense if the model of the delay in synaptic information processing is experimentally verified. Therefore, prospects must become projects.
Acknowledgements
This
paper is dedicated to Gerhard Werner (Department of Biomedical Engineering,
References
Akiskal HS. 1995. Mood
disorders: introduction and overview. In: Kaplan HJ,
Sadock BJ, editors. Comprehensive textbook
of psychiatry.
Williams and Wilkins. p 67-79.
American Psychiatric
Association. 1998. Diagnostic and statistical manual of
mental disorders. American Psychiatric
Association,
Araque A, Parpura V, Sanzgiri
RP, Haydon PG. 1999. Tripartite synapses: glia,
the unacknowledged partner. Trends
Neurosci 22: 208-215.
Auld DS, Robitaille R. 2003.
Glial Cells and Neurotransmission: An Inclusive
View
of Synaptic Function. Neuron 40: 389-400.
Barbas H, Zikopoulos B. 2007.
The prefrontal cortex and flexible
behavior. Neuroscientist 13: 532-45.
Bibring E. 1953. The mechanism
of depression. In: Greenacre P, editor.
Affective disorders.
Bezzi P, Volterra A. 2001. A
neuron-glia signalling network in the active brain.
Current Opinion in Neurobiology 11:
387-394.
Haydon PG, Araque A. 2002.
Astrocytes as modulators of synaptic transmission.
In: Volterra A, Magistretti PJ, Haydon PG,
editors. The tripartite synapse.
Glia in synaptic transmission.
Hobson JA, Scheibel AB. 1980.
Neurosciences research program bulletin.
Vol 18. The brainstem core: sensorimotor
integration and behavioral
state control.
Humphries MD, Gurney K,
in a small-world, not scale-free, network.
Proc R Soc B 273: 503-11.
systems. Transactions of the ASME 6: 290-4.
Kilmer WL, McCulloch WS, Blum
J. 1969. A model of the vertebrate
central command system. International
Journal of Man-Machine Studies
1: 279-309.
Krains SH. 1957. Mental
depressions and their treatment.
The MacMillan Company.
Lim H, Choe Y. 2006.
Compensating for neural transmission delay
using extrapolatory neural activation in
evolutionary neural networks.
Neural Information Processing – Letters
and Reviews 10: 147-61.
McCulloch WS. 1965.
Embodiments of mind.
McCulloch WS. 1966.
Commentary. In: Thayer L, editor. Communication:
theory and research.
Mitterauer B. 1983.
Biokybernetik und Psychopathologie. Das holophrene
Syndrom als Modell.
Mitterauer B. 1988. Computer
system for simulating reticular formation operation.
Mitterauer B. 1994.
Biokybernetik der Depression. Der informierte Arzt 1: 50-7.
Mitterauer B. 1998. An
interdisciplinary approach towards a theory of
consciousness. BioSystems 45: 99-121.
Mitterauer B. 2004. Imbalance
of Glial-Neuronal Interaction in Synapses: A
Possible Mechanism of the Pathophysiology
of Bipolar Disorder.
Neuroscientist 10: 199-206.
Mitterauer B, Kopp C. 2003.
The self-composing brain: Towards a glial-
neuronal brain theory. Brain and Cognition
51: 357-367.
of risk factors: global burden of disease
study. Lancet 349: 1436-42.
Peirce CS. 1931-1935.
Collected papers. Hartshome C, Weiss P,
editors.
Poewe W. 2007. Depression in
Parkinson’s disease. J Neurol 254 (Suppl 5) 49-55.
Rothman DL, Lebon V, Shulman
RG. 2002. Functional imaging studies of
neuron-glia transmitter cycling and
metabolic coupling in the living brain.
In: Volterra A, Magistretti PJ, Haydon PG,
editors. The tripartite synapse.
Glia in synaptic transmission.
Rothuber H, Kralovec K, Yazdil
K, Mitterauer B. 2007. Loss of self-understanding:
a behvaior-oriented model of depression.
Med Sci Monit 13: CR-CR 7.
74-78.
Smit AB, Syed NI, Schaap D,
van Minnen J, Klumperman J, Kits KS, and
others. 2001. A glia-derived
acetylcholine-binding protein that modulates
synaptic
transmission. Nature 411: 261-268.
Steriade M. 1996. Arousal:
revisiting the reticular activating system.
Science 272: 225-26.
Walter U, Prudente-Morissey L,
Herpertz S, and others. 2007.
Relationship of brainstem raphe
echogenicity and clinical findings in
depressive states. Psychiatric Research:
Neuroimaging 155: 67-73.
Werner G. 2007. Perspectives
on the neuroscience of cognition and
consciousness. BioSystems 87: 82-95.
Williams LM, Gordon E. 2007.
Dynamic organization of the emotional
brain: responsivity, stability, and
instability. Neuroscientist 13: 349-70.
Zinterhof P. 2006. Biomimetic simulation of brain functions. Thesis.
Zinterhof P. 2007. Computer simulation of the principle of redundancy of
potential command (unpublished).
Dr. Bernhard Mitterauer
Ignaz-Harrer
Strasse 79, A-5020
Telephone: +43 662 8044-3850, Telefax: +43 662 8044-3860
e-mail: bernhard.mitterauer@sbg.ac.at
[ BWW Society Home Page ]
© 2008 The Bibliotheque: World Wide Society