Medicine: Psychiatry:
Non-Feasible Intentional Programs
In Major Depressive Disorder:
Possible Role of Personality Structure
by Dr. Bernhard J. Mitterauer
Voltronics-Institute for Basic Research, Psychopathology and Brain Philosophy
Prof .em of Neuropsychiatry, University of Salzburg
Wals, Austria
Abstract:
A hypothetical model of the etiopathology of major depression is outlined. It is based on imbalanced information processing in tripartite synapses and the glial networks. Epigenetic factors may mainly be responsible for a hyperintentional personality structure of persons inclined to depression. Non-feasible intentional programs in the environment generate a stressful inner and outer situation that persists, because of a permanent striving for the realization of something that cannot be realized. Consequently, normal modes of behavior (e.g. sleeping, working etc.) are displaced and a loss of self-understanding occurs.
Key words: major depression, personality structure, hyperintentionality, stress, synaptic imbalance.
The etiopathophysiology of major depressive disorder is still unknown. The outline of the model proposed here may provide a new perspective towards elucidating this worldwide disorder. My hypothesis is as follows:
If a person with high aspirations is unable to realize an intentional program and is lacking a subjective explanation for this, a stress situation is generated. A feeling of impotence can activate a depressive episode caused by imbalances in tripartite synapses and dysregulations in the glial networks leading to impairments in action and cognition. As previously formally described (Mitterauer, 2004; 2010), intentional programming may be generated in the glial network modifying synaptic information processing. The typical personality structure susceptible to depression may be mainly determined by an epigenetic process and education. Since this personality structure persists, one can speak of hyperintentionality.
In an epigenetic process certain genes are expressed in a parent-origin-specific manner. Behavioral epigenetics attempts to provide a framework for understanding how the expression of genes is influenced by experience and environment (Champagne and Mashoodh, 2012) in producing individual differences in behavior (Zhang and Meaney, 2010), cognition (Powledge, 2011), personality (Bagot and Meaney, 2010), and mental disorders (Stuffrein- Roberts et al., 2008).
Although there are multiple routes through which parents can influence their offspring, recent studies of environmentally induced epigenetic variation have highlighted the role of non- genomic pathways. Epigenetic modifications can be identified through one or more forms such as DNA methylation, histone modifications, non-coding RNAs, chromosomal instability, transposons, and loss of imprinting (Kubota et al., 2012). Although there is increasing evidence for the role of epigenetic factors in explaining the effects of parental experience on offspring phenotype, it remains difficult to disentangle the effects of genetic variation and mutations from epigenetic modifications in perpetuating parental effects (Curley et al., 2011). Importantly, epigenetic modification of gene expression provides a mechanism for understanding the link between long-term effects of adverse life events and the changes in gene expression that are associated with depression (Dalton et al., 2014).
It is frequently observed that parents of children susceptible to depression are convinced of having a genius daughter or son who will make “great things” and innovations, which Bibring (1951) called high aspirations of persons susceptible to depression. For instance, genius artists and scientists are suddenly incapable of working without a conceivable reason, in the sense of a loss of self-understanding (Mitterauer, 2009; Rothuber et al., 2007). Personally I have treated a great computer scientist who saw a novel computer system “before his eyes”, but who was incapable of communicating it for technical implementation.
Pathophysiological findings with regard to the glial system mainly concern the astrogliopathology (Banasr et al, 2010; Verkhratsky et al., 2014), the role of microglia in inflammation (Brites and Fernandes, 2015), and the glial network (syncytium) where gap junction dysfunctions are found (Sun et al., 2012; Quesseveur et al., 2015). What the susceptibility to depression concerns, phenotype emerges as a final common outcome of diverse processes, called equifinality of disease susceptibility and stress. Most importantly, Spijker and coworkers (2010) elaborated biomarkers for major depressive disorder with moderate heritibility. In the model proposed dysregulations in the glial networks may affect imbalances in tripartite synapses and vice versa. Quesseveur and coworkers (2015) found that the degree of connexin 43 phosphorylation influences the expression of connexin proteins with different effects on overexpressed and underexpressed astrocytic gap junction networks. This mechanism may be fundamental for imbalances in tripartite synapses and their networks. There is some experimental evidence for overexpression of astrocytic receptors in Alzheimer’s disease (Yu et al., 2012) and temporal lobe epilepsy (Notenboom et al., 2006; Steinhäuser et al, 2015). Admittedly, in the case of major depression we still lack experimental evidence concerning overexpression of astrocytic receptors. Considering Parkinson’s disease the model proposed may provide some explanatory insight to the reason why the decreased production of neurotransmitter substances is associated with depression in about a third of patients (Barone, 2011). I suppose that the synaptic imbalance may be co-determined by an overexpression of astrocytic receptors underlying the depression of these patients.
The question arises as to how non-feasible intentional programs activate genetic susceptibility to depression. Generally, genome-wide association and linkage results provide constraints on allele frequencies and effect sizes of susceptibility loci (Flint and Kendler, 2014). Stress is caused by both environmental factors and factors within the nervous system. Patients with high genetic risk (Pritz and Mitterauer, 1984) frequently experience depressive episodes without major environmental stressors (Kendler et al., 2013). One can also say that in the case of non-feasible intentional programs the environmental situation is both stressful and inappropriate, since the patient himself generates this stress situation, unable to adapt his/her intentions to possibilities in the environment. If this inner stress persists, intentional programs operate hyperintentionally, permanently striving for realizing what cannot be realized. Such persons have a hyperintentional personality structure.
From a pathophysiological point of view, the model proposed seems to be mainly speculative. However, the model has been formally described with regard to synaptic imbalances (Mitterauer, 2004; 2010; 2015 a), intentional programming (Mitterauer, 2007) and the generation of modes of behavior in the brain stem (Mitterauer, 2015 b). These formal descriptions and interpretations enable computer simulations and exact designs for experimental investigation of the pathophysiology of major depressive disorder.
Most importantly, we may better understand that persons with depressed mood are permanently striving for something that is not feasible. This hyperintentionality is janusfaced: on the one hand intentional programs cannot be good enough, on the other hand the environmental situation is inappropriate. Such persons live in a troublesome stress situation. The interplay between the elementary components responsible for depression is shown in figure 1. Hyperintentionality and its non-feasibility generates stress that may cause a prolonged synaptic information processing. Since the networks of the brain do not function in real time, the repertory of the modes of behavior is displaced by the dominance of one or more behaviors over a prolonged duration. A person affected with this psycho biological disorder is unable to understand his (her) situation despite explanatory attempts from others losing the self-understanding. The loss of self-understanding negatively feeds back to hyperintentionality, since coping mechanisms are not available. I speak of a “diabolic cycle of depression” (Mitterauer, 2009).
References:
Araque, A., Carmignoto, G., Haydon, P.G., Oliet, R., et al. (2014). Gliotransmitters travel
in time and space. Neuron 81, 728-739.
Bagot, R.C. and Meaney, M.J. (2010). Epigenetics and the biological basis of gene x
environment interactions. Journal of the American Academy of Child and Adolescent
Psychiatry 49, 752-771.
Banasr, M., Chowdhury, G.M., Terwilliger, R., Newton, S., et al. (2010). Glial pathology
in an animal model of depression: reversal of stressinduced cellular, metabolic and
behavioral deficits by the glutamate-modulating drug riluzok. Mol. Psychiatry 15,
501-511.
Barone, P. (2011). Treatment of depressive symptoms in Parkinson’s disease. Eur. J. Neurol.
18, Suppl. 1, 11-15.
Bibring, E. (1953). The mechanism of depression. In: Greenacre, P. (ed.), Affective
disorders. Int. Univ. Press, New York, pp. 13-28.
Brass, M. and Haggard, P. (2008). The what, when, whether model of intentional action.
Neuroscientist 14, 319-325.
Brites, D. and Fernandes, A. (2015). Neuroinflammation in depression: microglia activation,
extracellular microvesicles and micro RNA dysregulation. Front. Cell Neurosci 9, 476,
doi:10.3389/fncel.2015.00476.
Champagne, F.A. and Mashoodh, R. (2012). Genes in context: Gene-environment interplay
and the origins of individual differences in behavior. Current Directions in Psychological
Science 18, 127-131.
Curley, J.P., Mashoodh, R., and Champagne, F.A. (2011). Epigenetics and the origin of
paternal effects. Horm. Behav. 56, 306-314.
Dalton, V.S., Kolshus, E., and McLoughlin, D.M. (2014). Epigenetics and depression: return
of the repressed. J. Affect. Disord. 155, 1-12.
Flint, J., and Kendler, K.S. (2014). The genetics of major depression.
http://dx.doi.org/10.1016/j.neuron.2014.01.027.
Greck de, M., Wang, G., Yang, X., Wang, X., et al. (2012). Neural substrates underlying
intentional empathy. SCAN 7, 135-144.
Kendler, K.S., Aggen, S.H., and Neale, M.C. (2013). Evidence for multiple genetic factors
underlying DSM-IV criteria for major depression. JAMA Psychiatry 70,
599-607.
Kraines, S.H. (1957). Mental depressions and their treatment. The Macmillan Company,
New York.
Kubota, T., Miyake, K., and Hirasawa, T. (2012). Epigenetic understanding of gene-
environment interactions in psychiatric disorders: a new concept of clinical genetics.
Clinical Epigenetics http://doi:10.1186/1868-7083-4-1.
Mitterauer, B. (2004). Imbalance of glial-neuronal interaction in synapses: a possible
mechanism of the pathophysiology of bipolar disorder. Neuroscientist 10,
199-206.
Mitterauer, B. (2007). Where and how could intentional programs be generated in the
brain? A hypothetical model based on glial-neuronal interactions. BioSystems 88,
101-112.
Mitterauer, B.J. (2009). Narziss und Echo. Ein psychobiologisches Modell der Depression.
Springer, Vienna, New York.
Mitterauer, B.J. (2010). Synaptic imbalances in endogenous psychoses. BioSystems 100,
113-121.
Mitterauer, B.J. (2015a). Balancing and imbalancing effects of astrocytic receptors in
Tripartite synapses. Common pathophysiological model of mental disorders and
epilepsy. MEHY 84, 315-320.
Mitterauer, B.J. (2015b). Model of the reticular formation of the brain based on
glial-neuronal interactions. Cogn. Comput. 7, 64-73.
Naus, C.C. and Giaume, C. (2016). Bridging the gap to therapeutic strategies based on connexin/pannexin biology. J. Trausl. Medicine, doi: 10, 1186/s 12967-016-1089-0.
Notenboom, R.G., Hampson, D.R., Jansen, G.H., van Rijenz, P.C., van Veelen, C.W., et al.,
(2006). Upregulation of hippocampal metabotropic glutamate receptor 5 in temporal
lobe epilepsy patients. Brain 129, 96-107.
Powledge, T. (2011). Behavioral epigenetics: how nurture shapes nature. BioScience 61,
588-592.
Pritz, W., and Mitterauer, B. (1984). Bipolar mood disorders: an affected sibling study.
I. Genetic background and course of illness. Psychopathology 17, 67-79.
Quesseveur, G., Portal, B., Basic, J.A., Ezory, P., et al. (2015). Attenuated levels of
hippocampal connexin 43 and its phosphorylation correlate with antidepressant-
and anxiolytic-like activation in mice. Front. Cell Neurosci.
http://doi.103389/fncel.2015.00490.
Rothuber, H., Kralovec, K., Yazdil, K. and Mitterauer, B. (2007). Loss of self-understanding:
a behavior-oriented model of depression. Med. Sci. Monit. 13: CR1-CR7.
Rothuber, H., and Mitterauer, B.J. (2011). Comprehensive behavioral analysis of patients
with a major depressive disorder. Med. Sci. Monit. 17, CR1-CR6.
Schel, M.A., Kühn, S, Brass, M., Haggard, P., Ridderinkhof, K.R., and Crone, E.A. (2014).
Neural correlates of intentional and stimulus-driven inhibition: a comparison.
Front. Hum. Neurosci. doi:10.3389/fnhum.2014.00027.
Spijker, S., Van Zanten, J.S., De Jong, S., Pennix, B.W., et al. (2010). Stimulated gene expression profiles as a blood marker of major depressive disorder. Biol. Psychiatry 68, 179-8b.
Steinhäuser, C., Grünnet, M. and Carmignoto, G. (2015). Crucial role of astrocytes in temporal lobe epilepsy. Neuroscience 323, doi: 10.1016/ j.neuroscience. 2014, 12.047.
Stuffrein-Roberts, S., Joyce, P.R., and Kennedy, M.A. (2008). Role of epigenetics in
mental disorders. The Australian and New Zealand Journal of Psychiatry 42,
97-107.
Sun, J.D., Liu, Y., Yan, Y.H., Li, J. and Chen, N.H. (2012). Gap junctions dysfunction in
the prefrontal cortex induces depression-like behaviors in rats. Neuropsychopharm.
37, 1305-1320.
Verkhratsky, A., Rodriguez, J.J. and Steardo, L. (2014). Astrogliopathology: a central
element of neuropsychiatric diseases. Neuroscientist 20, 576-588.
Yu, W., Mechawar, N., Krantic, S., Chabot, J., and Qurion, R. (2012). Upregulation of
astrocytic α7 nicotinic receptors in Alzheimer’s disease brain – possibly relevant
in amyloid pathology. Mol. Neurodegener. 7 (Suppl. 1), 07.
Zhang, T.Y., and Meaney, M.J. (2010). Epigenetics and the environmental regulation of
the genome and its function. Ann. Rev. Psychol. 61, 439-466.
Acknowledgement:
The author is very grateful to Marie Motil for preparing the final version of the paper.
To contact the author: Email: mitterauer.b@gmail.com
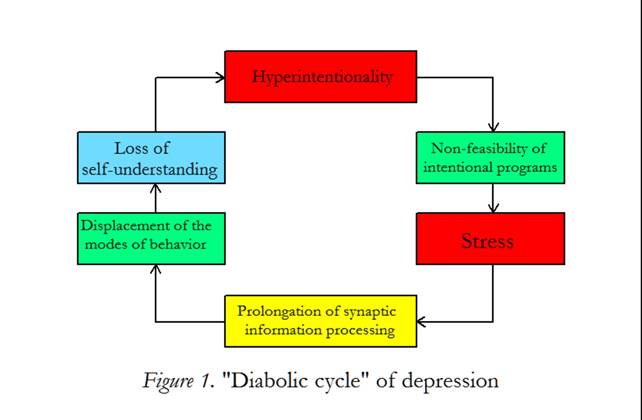
Figure 1: “Diabolical Cycle” of depression. Hyperintentionality causing non-feasibility of intentional programs generates an inner and outer stress. This stress situation is responsible for a prolongation of synaptic information processing. On the behavioral level, normal modes of behavior (eating, working, sleeping etc.) cannot be generated in real time so that the biopsychical behavior is displaced. The patient cannot self-explain his (her) impaired situation and a loss of self-understanding occurs. (The components of the cycle are interconnected by arrows.)