The Significance of Proving the
Non-existence of
Heptachloro-1,4-Dioxane from Melting Point
122oC
By Prof Dr Randolph
Riemschneider, LBFel
And the Central Institute of Chemistry, Universidade
Federal de Santa Maria (UFSM),
Santa Maria, Rio Grande do Sul, Brazil
In an article in this journal in August of last year, the author reported that the second heptachloro-1,4-dioxane isomer (I), the one from m p 122oC, described in the 50s does notexist (1), as proved by numerous experiments by our working group in 1947 - 88 (2): preparation of 1,4-dioxane and Cl-substituted dioxane chlorinations (Tab 1) began in 1947 (3.4).
On the strength of it there were several questions, in particular about the significance of this result in which 40 years of experimental have been invested (2,3).
ANSWER: Isolating two I-isomers melting at 57 and 122oC would have meant that there would for the first time have been two chair configurations in a conversion relationship to one another, seperated in substance: a case of isomerism of a chair configuration pair whose partners are present as crystals. The theoretically possible I-isomers are formulated in fig. 1 (1.9). We have been interested in such cases of isomerism since 1947 (3)[1]).
Only after many years of effort did the author suceeded in
experimentally proving such an isomerism (5a) by synthesizing two monofluoro-endecachloro-cyclohexane (II)
isomers separated in substance . The constitution and configuration of II
were proved jointly with Prof Y Morino of
Fig 1: The chair configuration partners 6a(H)2ea3ea5ea6e(Cl) and 6e(H)2ea3ea5ea6a(Cl) of 2.2.3.3.5.5.6-heptachloro-1,4-dioxane
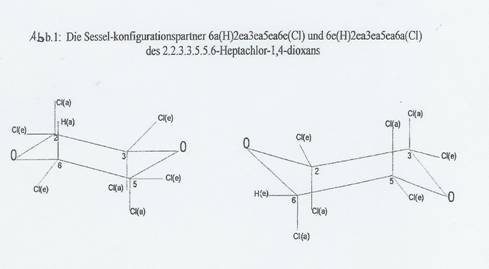
Fig 2: The chair configurations 1e(F)1a2ea3ea4ea5ea6ea(Cl) and 1a(F)1e2ea3ea4ea5ea6ea(Cl) of monofluoro-endecachloro-cyclohexane C6FCl11 (6,5)
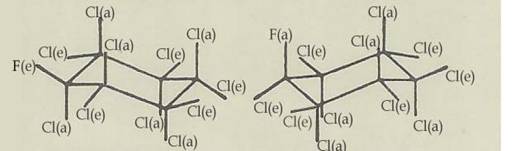
Key to Fig 1 and 2:
In an assumed conversion of the 1,4-dioxane-5 chair, in which eg the C-atoms 2 and 6 and the O-atom 4 migrate down and the C-atoms 3 and 5 and the O-atom 1 up, two configurations of 1,4-dioxane substitution products change into each other, which we combine to a chair configuration pair. The pair's original configuration is known as the "body", the converted one as the "antibody". In the case of 2.2.3.3.5.5.6-heptachloro-1,4-dioxane (I), the body's configuration is 6a(H)2ea3ea5ea6e(Cl) and that of the "antibody" 6e(H)2ea3ea5ea6a(Cl), ie all the e-bonded substituents change into a-bonded ones on conversion, and vice versa [e for equatorial, a for axial (9,10)].
In the case of monofluoro-endecachloro-cyclohexane, the body's configuration is 1e(F)1a2ea3ea4ea5ea6ea(Cl) and the antibody's 1a(F)1e2ae3ae4ae5ae6ae(Cl), ie all the e-bonded substituents change into a-bonded ones on conversion, and vice versa: Diag 2.
For energetic reasons there was no great probability of two heptachloro-1,4-dioxane isomers, C4HO2Cl7, existing as the two conversion partners differ by one Cl-H position [mol wts in ratio 35.5:1]. The case of monofluoro-endecachloro-cyclohexane, C6Cl11F, we realized - prepared in various ways [(5) and PROJECT II 6 (10)] - is energetically more favourable: difference in Cl-F position [mol wts in ratio 35.5 : 19].
As it was a question of a professorial thesis that erroneously described the case of isomerism concerned, immediate official objections and steps on the part of the author would certainly have had consequences for the "discoverer", particularly he (7) was not able to deliver the I-isomer from m p 122oC on request. We did not want to become involved immediately and only to publish after concluding our own experiments. It was also a question of not exposing Prof Freudenberg, the principal referee for the professorial thesis.
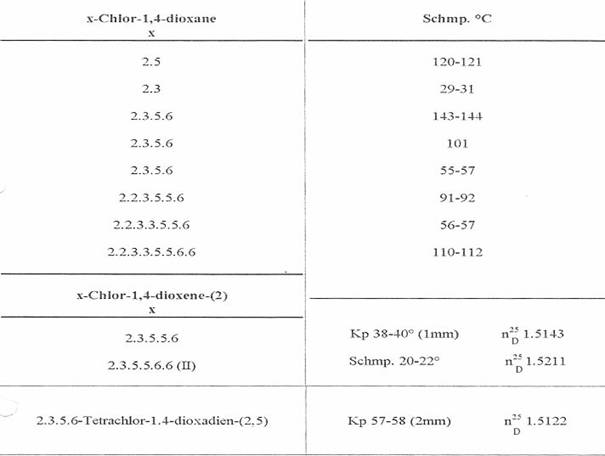
We had immediately doubted the existence of I from m p 122oC the first time we heard of it in a lecture (8). Together with K Lohmann, W Gericher, W Cohnen, A Heymons, we had already isolated octachloro-1,4-dioxane from m p 110 - 112oC (III), among other dioxane chlorination products, in our lab in 1947:
Tab 1 (3). It seemed improbable, after our extensive experience in the field of Cl-substituted cyclohexanes, that a symmetrically built III (dipole moment: 0) would melt at a lower temperature than a I-isomer. Reference to a discussion following a lecture at the Innsbruck Chemists Congress on 01 Apr 53 at (8).
The search for a second I-isomer, begun in 1947, led to examining the chemical behaviour of the I isomer from 57oC more closely, eg HCl splitting off from I from 57oC led to 2,3,5,5,6,6-hexachloro-1,4-dioxene from m p 20-22oC and this in its turn opened up new paths for getting to Cl-F substituted dioxanes and dioxenes: Tab 2 (11,12,14).
The isomerism of monofluoro-heptachloro-1,4-dioxane (IV) was included in the experiments in the investigation described above, its theoretically possible chair configurations are also represented in Fig 1, read there: F instead of H. Here too, however, we only succeeded in isolating and identifying one isomer - as with I - in 1954 [1964-72 (13a,b)].
Details of the insecticidal activity of some halogenated 1,4-dioxanes in Tab 3 (14).
Table 1:
10 isolated Cl substituted 1,4-dioxanes and -dioxenes [1947-49 (3,10)]
Based on the data established for the
insecticidal action of heptachlorodioxane (I) from m p 56-57oC (1,
6, 7, 8 and Tab 3), it cannot be classified as an insecticide that can compete
with DDT or Gammexan
1954 addendum:
K Hayduk and M Lüdecke [Z Naturforschg 11
B 383 (1954)] concerned themselves with the "physical factors of the
action of heptachlorodioxane on Drosophila", but without
characterizing the product more closely: no details of melting point, no
reference to "isomers".
Table 2:
F- and Cl-substituted 1,4-dioxanes, -dioxenes and -dioxadienes, starting from heptachloro-1,4-dioxane (I) from m p 56-57oC and 2,3,5,6,6-hexachloro-1,4-dioxene (II) from 20-22oC (1951-53)
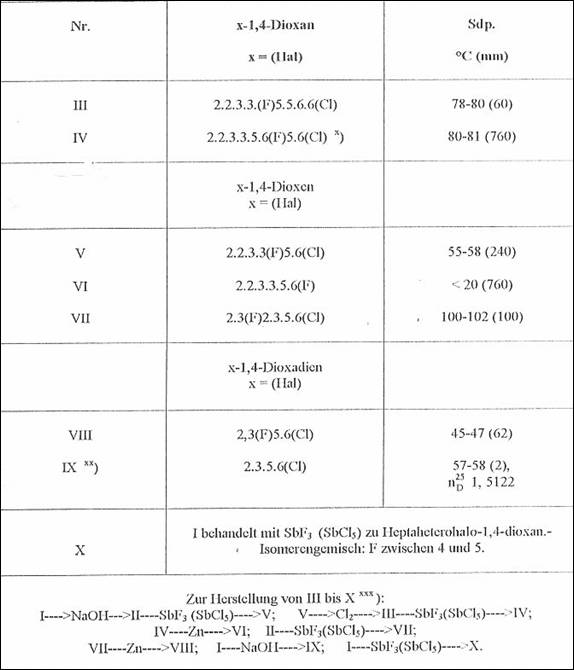
x) Liquid mixture of isomers of conversion partners not suited for seperating in the sense of the problem formulated in Fig 1
xx) Cf bottom Tab 1
xxx) NaOH treatment in CH3OH, zinc dust treatment, chlorination in stages and fluorination in SbF3, adding SbCl* [no added solvent] as well as with HgF2
Table 3:
The contact-insecticidal activity of halogenated saturated and unsaturated dioxanes (Tab 1, 2) on Calandra granaria in filter test, avoiding respiratory poison action (contact poison test apparatus II)X)

x) Contact poison test apparatus II (10): Avoiding respiratory poison action by a current of air stream flowing from top to bottom through closed sintered-glass suction filters in parallel. Insects and test substances: in each filter there were 30 Calandra granaria on filter disks containing the substances for testing (applied in acetone solution of defined concentration, dried on pin stands). The halo com-pounds in Tab 1, 2 not listed in this table proved ineffective in these conditions.
xx) Relative activity when using 500μg/cm2
References:
(1) R Riemschneider
About so-called
heptachloro-1,4-dioxane from m p 122oC (Über das sogenannte Heptachlor-1,4-dioxan vom
Schmp 122oC)
The Bi-Monthly Journal of The BWW Society, Aug 2005
http://www.bwwsociety.org/journal/html/bcompounds.htm
(2) loc cit (3,4,) and [(10): (656-659, 669-674, 677, 678)]
Authors: R Riemschneider with (in chronological order): K Schürken, K Lohmann, W Gerischer, W Cohnen, A Heymons, F Scherer, G Seeliger, R Wasicky, W Stuck, E B Grabitz, D Takei, P Nowack, H Fereira, M Azhar (1947-86) and with M M Faria, H S Chang, Chemical-Engineering-Development (1977-82); cf (4c)
(3) R Riemschneider, K Lohmann,
W Gerischer, W Cohnen, A Heymons (1947-49)
About preparing and
identifying 10 Cl-substituted 1,4-dioxanes, -dioxenes and subsequent products
as well as testing them for contact-insecticidal activity (direct chlorinations
of 1,4-dioxane and 1,4-dioxane chlorinated pro-ducts);
2,2,3,3,5,5,6-heptachloro-1,4-dioxane from m p 56-57oC and
octachloro-1,4-dioxane from m p 110-112oC (Über die Herstellung und Identifizierung von
10 Cl-substituierten 1,4-Dioxanen, - Dioxenen und Folgeprodukten sowie ihre
Prüfung auf kontakt-insektizide Wirksamkeit (direkte Chlorierungen von
1,4-Dioxan und 1,4-Dioxan-Chlorierungsprodukten);
2,2,3,3,5,5,6-Hepta-chlor-1,4-dioxan vom Schmp 56-57oC,
Oktachlor-1,4-dioxan vom Schmp 110-122oC)
Bull Inst of Physiolog Chem Berlin, 15 p, Jan 49, secreted at the instigation of Dr F Scherer, Farbwerke Hoechst AG; cf loc cit (10): (553); cf lect of Apr and May 52 in Berlin and Vienna, loc cit (10): (446)
(4) Loc cit (2) eg
a) R Riemschneider, A Heymons, G Seeliger, E B Grabitz (1954-56)
Experimental testing of question of existence of second "heptachloro-1,4-dioxane" (m p 122oC) (Experimentelle Prüfung der Frage der Existenz des zweiten "Heptachlor-1,4-dioxans" (Schmp 122oC))
Ms 10 p, 1956. Unpublished experiments in Dept of Biochem, FU Berlin and G Seeliger's 30-page degree thesis, Tech Univ of Berlin, 1956
Despite the most intensive efforts, it proved impossible to isolate a second heptachloro-1,4-dioxane isomer by the time the manuscript was written, or indeed later.
(b) R Riemschneider (lecturer), E B Grabitz, J Takei, P Nowack (1958-62)
Continuing experimental work in search of two 2,2,3,3,5,5,6-hepta-chloro-1,4-dioxane isomers in 1,4-dioxane chlorinated products (starting from 1,4 -dioxane as well as defined tetra- and hexachloro-1,4-di-oxanes and from dioxadiene) (Fortsetzung der experimentellen Arbeiten zur Suche nach zwei 2,2,3,3,5,5,6-heptachlor-1,4-dioxan-Isomeren in 1,4-Dioxan-Chlorierungsprodukten (ausgehend von 1,4-Dioxan sowie defin-ierten Tetra- und Hexachlor-1,4-dioxanen und vom Dioxadien)
Lecture in colloquium of Dept of Biochem, FU Berlin on 20 Jan 61
Lab reports from 1958-62 from above dept. Result up to 1986: negative. There is only one heptachlorodioxane isomer [(680) in (10)]
(c) R Riemschneider, M M Faria, H
Continuing 1,4-dioxane chlorination experiments. The heptachloro-1,4-dioxane problem (Fortsetzung der 1,4-Dioxan-Chlorierungsver-suche. Das Heptachlor-1,4-dioxan Problem) (commissioned research)
24-page report, 1983, of experiments conducted by
Chemical-Engineering -Development of S
The non-existence of so-called heptachlorodioxane from m p 122oC was also confirmed by this independent institution.
(5) R Riemschneider, W Pollack, F R Pesserl et al (1969-76)
(a) Long-term chlorination experiments to isolate monofluoroendeca-chlorohexane(s), starting from fluorobenzene, difluorobenzenes, mo-nofluorotrichlorobenzene, pentachloromonofluorobenzene, mono-fluoroheptachlorocyclohexane from m p 218oC, from monofluorhexachlorocyclohexane oils and many other fluorine derivatives (Langzeit-Chlorierungsversuche zur Isolierung von Monofluorendekachlorcyclohexan(en), ausgehend von Fluorbenzol, Difluorbenzolen, Monofluortrichlorbenzol, Pentachlormonofluorbenzol, Monofluorheptachlorcyclohexan vom Schmp 218oC, von Monofluorhexachlorcyclohexan-Ölen und vielen anderen Fluorderivaten)
PROJECT II 6 (10)
(b) Negative results with respect to the further
search for a second heptachloro-1,4-dioxane isomer in chlorinated products of
tetra- and hexachloro-1,4-dioxanes. (Negative Ergebnisse hinsichtlich der weiteren Suche nach einem zweiten
Heptachlor-1,4-dioxan-Isomeren in Chlorierungsprodukten von Tetrachlor- und
Hexachlor-1,4-dioxanen)
Ms, 12 p, 1976. Experiments from the Central Inst of Chem, Univ Fed of Santa Maria (UFSM), S Maria, RG, Brazil.
The long-term chlorinations were conducted in Carius
(bomb) tubes that were exposed to sunlight on the institute roof for up to two
years. Of 300 tubes, some 180 were lost through explosion. Such experiments
were no longer possible in
(6) Y Morino, R Riemschneider (lecturer), M Z Azhar, W Plieth
X-ray of 2 C6FCl11 isomers
Report, 1986, 9 p; lecture given in Inst of Chem, SAITAMA Univ of Urawa, Japan on 15 May 86. Loc cit [(10): Project II (211-212, 214-215)]
(7) W Stumpf
Chemistry and applications of 1,4-dioxane (Chemie und Anwendungen des 1,4-Dioxans)
Verlag Chemie GmbH, 1956, p
130-133
(8) F
Scherer, R Riemschneider (discussant), R Wasicky
Critical comments during
discussion of lecture by W Stumpf on "Dioxane-chlorinated products as
insecticides" (Kritische
Diskussionsbemerkungen zum Vortrag von W Stumpf über
"Dioxan-Chlorierungsprodukte als Insektizide")
Chemists Congress,
Text of comments sent to Österreichische
Chemiker-Zeitung,
(9) R Riemschneider
About the configurations of 1,4-dioxane substitution products (Über Konfigurationen von 1,4-Dioxan-Substitutionsprodukten)
Z Naturforschg 8B 745-751 (1954), Free
(10) R Riemschneider
Re-reading 66 years of chemistry (Nachlese, 66 Jahre Chemie) in preparation
PROJECT I - XXVI
(11) R
Riemschneider (lecturer), W Stuck, W Gerischer
"Cl- and F-substituted 1,4-dioxanes, -dioxenes and -dioxadienes"
Lecture given in chemical colloquium, Farbwerke HOECHST, in Höchst in Nov 53 (chair: Dr F Scherer, director): tab 1,2, and 3
Tables 1,2, and 3 were presented as slides during the lecture and then distributed subsequent to it.
From the lecture: The most important halo compounds synthesized by us, which can be derived from 1,4-dioxane and its unsaturated forms, are listed in the said tables.
Here, too, we considered the topic "Constitution (configuration) and action". But only a few of the forms based on the dioxane chair model displayed any contact-insecticidal activity at all, and than only moderately, viz the supersubstituted compounds: heptachlorodioxane and fluorohexachlorodioxane and very weak action the octachloro-substituted ones: Tab 3.
All of the 1,4-dioxane chlorinated products with fewer than 7 halogen atoms proved to be inactive. The same also applies for the Cl- and F-substituted 1,4-dioxenes and 1,4 dioxadienes, whose spatial structures differ substantially from the saturated forms.In collaboration with the Crop Protection Dept, Farbwerke HOECHST, most of the halo compounds listed in tab 1 and 2 were tested for insecticidal, fungicidal and acaricidal activity in field trials over several years, but unfortunately without positive results.These investigations were only possible because HOECHST prepared 7 kg of heptachloro-1,4-dioxane and 2.5 kg of 2,3,5,5,6,6-hexachloro-1,4-dioxene-(2) according to our instructions in its Technikum in 1951. The latter compound was obtained elegantly from heptachlorodioxanes and served as the starting material for getting into the chemistry of the said flourinated chloro-dioxanes, -dioxenes and dioxadienes.
(12) R
Riemschneider, F Scherer, W Stuck
Preparing fluorinated chloro-1,4-dioxanes, -dioxenes, dioxadienes and testing for contact-insecticidal activity
Ms 1953, 17 p (secreted): Tab 1, 2
Free Univ of
Experimental details in Special Part at X 3.2; cf (10), there (667 b, 675)
Ms was sent confidentially to Prof Dr S Takei, Univ of Kyoto. English translation of letter in reply below
Institute for Insect Control
01 Feb 54
Dear colleague Riemschneider:
Many thanks for sending your manuscript (secreted for Farbwerke Hoechst) with the results of "Chlorination and flourination of dioxane", including 2 tables: 10 isolated Cl-substituted 1,4-dioxanes and -dioxenes (1947-49) and F- and Cl-substituted 1,4-dioxanes, -dioxenes and -dioxadienes, starting from heptachloro-1,4-dioxane (I) from melting point 56-57oC and hexachloro-1,4-dioxene (II) from melting point 20-22oC (1951-53).
Please send, if possible, 50 mg each of some of the products. We have already received larger quantities of I from you for animal tests.
Confidentiality with respect to the manuscript assured.
I hope that my German from my
Cordial greetings
Your Takei
(13a) R
Riemschneider (1954)
Monofluoro-heptachloro-1,4-dioxane,
melting from 101oC - dipole moment: 0.2 D
(Monofluor-heptachlor-dioxan, schmelzend ab 101oC - Dipolmoment: 0,2
D)
Ms May 1954, FU Berlin
(13b) R Riemschneider, D Takei, P Nowack,
S G Fereira
Further
experiments to gain monofluoroheptachloro-1,4-dioxane isomeres
(Fortsetzung der Versuche
zur Gewinnung von Monofluor-heptachlor-1,4-dioxan-Isomeren)
Ms Oct 72 - experiments conducted 1964-72 in labs of
FUB and UFSM
(14) R.
Riemschneider
“Constitution
and activity of insecticides: Special structure of hepta chloro-1,4-dioxane and related compounds.”
Lecture,
given at the chemical colloquium of the central
Prepared
as manuscript for the Japanese periodical: Botyu-Kagaku
(Scientific
[1] There is a detailed description of the theoretically possible chair
configurations (constellations) of X-substituted 1,4-dioxanes elsewhere (9)
[ BWW Society Home Page ]
© 2006 The BWW Society/The Institute for the Advancement of Positive Global Solutions