Ways
Out of the BSE Crisis - Alternative Solutions
by Professor Dr.Dr.h.c.
Randolph Riemschneider, Life B.Fel.[1]
Instituto Central de Quimica da Universidade
Federal de Santa
Maria (UFSM), Santa Maria, RS, Brazil
Institute of Biochemistry, Free University of
Berlin (FUB), Germany
[Editor’s
Note: This paper is presented as Part II of a two-part series]
Keywords: Animal extracts: synthetic, cell culture based -
plant extracts: yeast, cereal, glycoprotein based - chemicytorrhysis - activation of cell metabolism,
fermentation and respiration increasing factor; (WARBURG tests), ASO- and
ASD-inhibition - applications in cosmetics, in medicine, as animal food additive,
as food drug - whitening effect - proteinfree extracts - trade
marks – BSE[2]
Owing to the pronounced increase in fermentation, the invention
therefore makes it possible to carry out an aerobic fermentation process where
a preparation of the invention is used to promote fermentation especially
during the initial phase.
In discussions with master brewers at several breweries, the author
learned that an acceleration of the brewing process is by no means desirable. A
question asked by the author in this respect was answered as follows:
"Dear Professor, we have time."
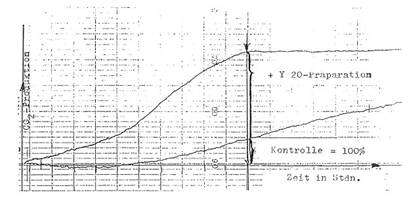
Automatic registration of fermentation:
We developed a special equipment
to control the production and quality of Y 20 - the basis for several
mentioned preparations – i.e. “Fermentationstest with automatic registration of the fermentation process under the
influence of Y 20”: Illustration 2. This simple and rel. quick method (9-11
hours) is superior to other tests like animal tests, manometry (WARBURG),
microbiology – and well to reproduce.
Registration curve in Diagram 5:
Illustration
2:
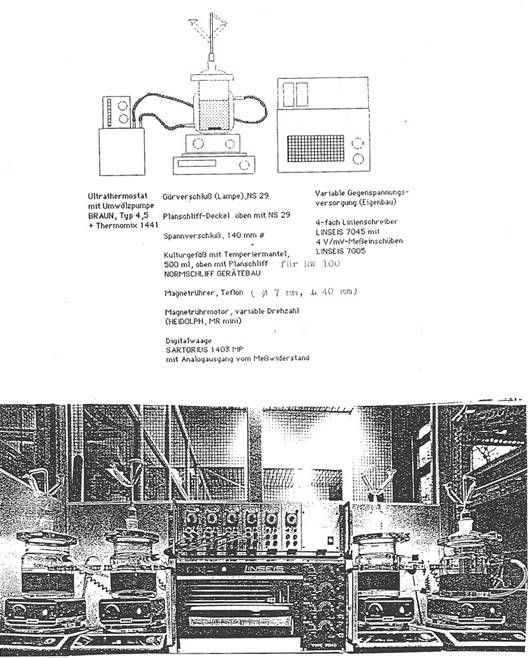
Procedure: Die 4 auf 30° C vorgewärmten, mit Rührstäben
gleicher Dimension versehenen Gefässe (1) bis (4) werden beschickt mit je 30,0g
Glukose, je 5,0g Caseionpepton, tryptisch verdaut (Merck), je 0,6g MgSO4•7H20,
je 450ml vorgewärmtes Leitungswasser. Nach Zugabe von 3,0g Y 20 pur in Gefäss
(1) und (2) und nach 15 Min. Zugabe 0,6g Trockenbackhefe in Gefäss (1) bis (4)
wird jeweils auf 500ml aufgefüllt. Dann
werden die Magnetrührer eingeschaltet und die Gefässe geschlossen. 0-Stellung
der Waage und Start aller Schreiber. Die Gefässe (3) und (4) sind Kontrollen: Kurve
2.
Beispiel in
Diagram 5.
Für die
Gefässe (1) und (2) wird die Zeit des Erreichens der maximalen CO2-Entwicklung
(Knickpunkt der Kurve 1) abgelesen; im allgemeinen nach 9-11 Stunden:
% Wirksamkeit
von Y 20 = (Knickpunkt der Kurve 1) : (Knickpunkt der Kurve 2) x 100
V 5.3 Claims
of the PR China-patent 91105975.1
in Chinese Plate 8a (document) in German Plate
8b (document)



re: claim 27, see V 5,4 re: claim 30, see V 6,1 and 6,2
V 5.4: ATP
formation (8a,b)[3]
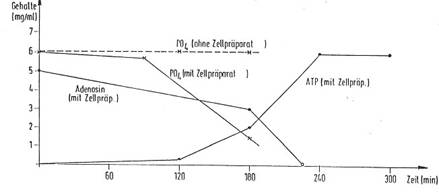
In Diagram 6a and b you find the chronological progress of ATP formation
in an incubated formulation (Diagram 6b in German language):
Plant cell preparations of the invention DE 4042157 C 2 (8a) show a surprising
and pronounced influence on ATP formation in suitable systems such as a yeast
suspension containing glucose and phosphate.
If a formulation of 1 g of baker's yeast, 4.0 ml of phosphate buffer
having a pH of 7.4, 0.1 ml of yeast cell preparation (prepared as in Example
1), 1 mg of magnesium gluconate and 0.1 ml of manganese sulfate are incubated
at 30°C for 60 minutes, and 2.0 ml of a solution of 30 mg of adenosine and 200
mg of glucose are then added and the mixture allowed to react at 37°C and a pH
value of 7.4 for up to 270 minutes, a significant yield of ATP is recorded
after some time, while the phosphate and adenosine content is reduced
accordingly. Diagram 5 shows the measurement results of the increased ATP
formation after 90', 120', 180', 240', and 270', all of the samples being
worked up and the resulting ATP yields, the adenosine quantities consumed and
the phosphate decrease being measured in each case. Without the addition of the
cell preparation of the invention, practically no ATP production is measured
under otherwise identical conditions (dotted line in diagram 5a,b).
Thus the invention using a plant cell preparation opens up a very simple
and commercially practicable way to biochemical ATP formation in a yeast
suspension medium to which adenosine and phosphate are added. Other nucleotides
such as GTP, CTP and TTP may be prepared analogously.
Diagram 6a:
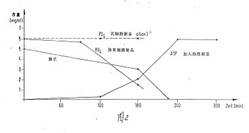
Diagram 6b:
V 6.1:
TLC-chromatograms from DE 40 42 157 C2, fig. 3-6
In Fig 3 resp Fig 5 there are the TLC of the identified amino acids resp
nucleo acid components of a yeast cell preparation prepared according to DE 40
42 157 C2. Fig 4 and 6: TLC of the
starting materials.
Fig 3a. amino acid TLC of a dialysate
Y 20 – starting material to develop the cosmetic additive CYTOCATALYZER;
cf. claim 30 in Plate 8b.
Key to Fig 3-6:

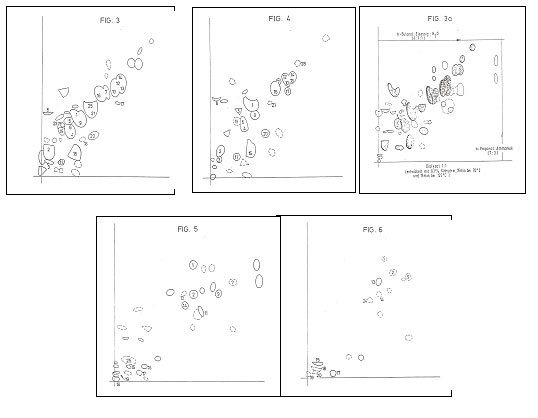
Experimental details in PROJ XXIII, “Re-reading- 66 years chemistry”
(20)
V 6.2:
CYTOCATALYZER – a yeast cell preparation based on Y 20
In the sections IV 1-3
several patents are described which are the basis for the production of
metabolic active plant cell preparations; in this case from yeast, Saccharomyces
we received the above mentioned starting material Y 20 which served to
develop the prolteinfree cosmetic additives called CYTOCATALYZER - in trade for many years in
Here follow Certificates1 and 2 of CYTOCATALYZER IKD and YSC:
Certificate 1: CYTOCATALYZER IKD
Analytical data:
Appearance
clear solution
Odor
deodorated
pH 6,7
dry
substance 4,1 %
N 0,4 %
metabolic activity
> 2,0
(WARBURG-factor)
Identification: amino
acids positive
(ninhydrin)
peptides positive
(biuret)
nucleo acid components
positive (TLC)
Puritiy: heavy
metals < 20 ppm
As < 2 ppm
sterility germ free
Certificate 2: CYTOCATALYZER - YSC
Analytical data:
Appearance
clear solution
odor deodorated
pH 6,7
dry substance
7,1 %
N 0,6 %
aminoacidoxydase- positive
inhibition
metabolic
activity > 2,0
(WARBURG-factor)
Identification: amino acids positive
(ninhydrin)
nucleo acid
components TLC
peptides positive
(biuret)
Purity: heavy
metals < 20 ppm
As <
2 ppm
sterility germ
free
The CYTOCATALYZER preparations
passed all toxicological and dermatological test without showing any
negative results, i.e. examinations like DL50 (mice), chronic toxicity
(rats), teratogenity (rabbits), tests according to TURNER, BÜHLER, DRAIZE
(eye,skin), PATCH, AMES. Tests explained in Table 6 in IV 4.
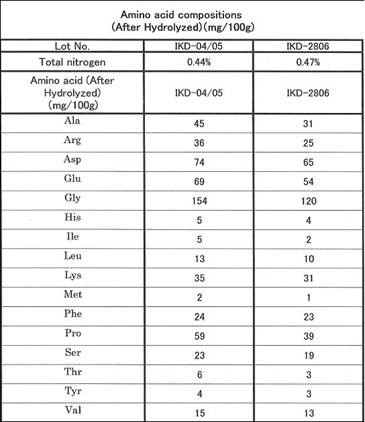
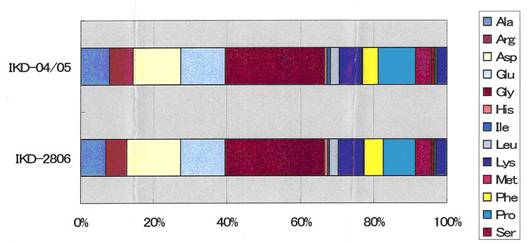
CYTOCATALYZER IKD - 16 amino
acids determined
VI APPENDIX
VI
1
Cosmetic composition with a whitening effect,
method for the production and use thereof (19)
EP 03 718 674.9 – PCT/EP 03/01874
from 24.2.2003 (inventor: the author) 28p, 15 claims in Plate 10,
Abstract:
The invention
relates to cosmetic compositions with a whitening effect, containing at least
one enol lactone of the following general formula (I in Plate 9) thus cited
and/or one or several tautomers thereof, also having the following formulae
(Ia-Ic) thus cited or a salt, especially an alkaline earth salt, alkali salt or
ammonium salt as an active ingredient. The invention also relates to a method
for the production and use thereof for preventing the formation of brown skin
spots and/or for lightening (whitening) or removing brown skin spots which are
already present, especially for treating melanoses in mammals including humans.
Plate 9: General
formula I and its tautomers Ia- Ic
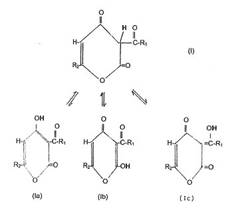
Plate 10: Claims (document)
1. Kosmetische Zusammensetzung mit"Whitening-Effekt", die als Wirkstoff mindestens ein Enol-Lacton der nachstehend angegebenen allgemeinen Formel (I in Plate 9) und/oder eines oder mehrere seiner Tautomeren der Formeln (la-ic) (la) (Ib) (Ic) worin Ri und R2 gleich oder voneinander sind, wobei Ri für Phenyl, C1-4-Alkyl-phenyl, insbesondere Benzyl, C1-4-Alkyl, C1-4-Alkenyl oder C14-Alkinyl steht, und R2 für COOH oder COOR (worin R die für Ri angegebenen Bedeutungen hat) oder für die gleichen Reste wie Ri steht, und worin die OH-Gruppen gegebenenfalls teilweise oder vollständig durch Halogenidreste, insbesondere Cl und/oder Br oder durch Oxyglycosid-, Thioglycosid-und/oder Aminoglycosid-Reste ersetzt sein können, und/oder ein oder mehrere Salze, insbesondere Erdalkaii-, Alkali-und/oder Ammoniumsalze, davon enthält.
2. Kosmetische Zusammensetzung nach Anspruch 1, dadurch gekennzeichnet, dass sie als Wirkstoff mindestens ein Enol-Lacton der in Anspruch 1 angegebenen Formeln (I), (la), (lob) und (Ic) enthält, worin Ri und R2 gleich oder verschieden sind und für CH3 oder C2H5 stehen.
3. Kosmetische Zusammensetzung nach einem der Ansprüche 1 bis 2, dadurch gekennzeichnet, dass sie als Wirkstoff 3-Acyl-3,4-dihydro-6-alkyl-2H- pyran-2, 4-dion der Formel (I) bzw. (la, lb, lc), worin Ri und R2 für CH3 oder C2H5 stehen, oder ein Salz, insbesondere ein Natriumsalz, desselben enthält.
4. Kosmetische Zusammensetzung nach einem der Ansprüche 1 bis 3, dadurch gekennzeichnet, dass sie den (die) Wirkstoff (e) der Formel (I) bzw.
(la, lb, Ic) in einerwirksamen Menge, vorzugsweise in einer Menge von 0,01 bis 5,0 Gew.-%, besonders bevorzugt in einer Menge von 0,1 bis 1,0 Gew.-%, speziell in einer Menge von 0,15 bis 0,5 Gew.-%, bezogen auf das Ge- samtgewicht der kosmetischen Zusammensetzung, enthält.
5. Kosmetische Zusammensetzung nach einem der Ansprüche 1 bis 4, dadurch gekennzeichnet, dass sie als zusätzliche (s) Additiv (e) Aldohexonsäuren, Ketohexonsäuren, Weinsäure, Citronensäure und/oder Ascorbinsäure enthält.
6. Kosmetische Zusammensetzung nach Anspruch 5, dadurch gekennzeichnet, dass sie die zusätzlichen Additive in einer Menge von 0,01 bis 5 Gew.-%, vorzugsweise in einer Menge von 0,1 bis 0,5 Gew.-%, bezogen auf das Gesamtgewicht der kosmetischen Zusammensetzung, enthält.
7. Kosmetische Zusammensetzung nach einem der Ansprüche 1 bis 6, dadurch gekennzeichnet, dass sie als weitere(s) Additiv(e) S-haltige Aminosäuren und/oder S-haltige Oligopeptide, insbesondere Glutathione, enthält.
8. Kosmetische Zusammensetzung nach Anspruch 7, dadurch gekennzeichnet, dass sie die weiteren Additive in einer Menge von 0,01 bis 3,0 Gew.- %, vorzugsweise in einer Menge von 0,1 bis 0,5 Gew.-%, bezogen auf das Gesamtgewicht der kosmetischen Zusammensetzung, enthält.
9. Kosmetische Zusammensetzung nach einem der Ansprüche 1 bis 8, dadurch gekennzeichnet, dass sie als weitere(n) Wirkstoff(e) keratolytisch wirksame Verbindungen, insbesondere Keratase und/oder alpha-Hydroxysäuren (AHA-Säuren), enthält.
10. Kosmetische Zusammensetzung nach Anspruch 9, dadurch gekennzeichnet, dass sie als weitere Wirkstoffe Rubiginsäure, Hydracetsäure, Comensäure, Chelidonsäure und/oder Mekonsäure enthält.
11. Kosmetische Zusammensetzung nach Anspruch 9 oder 10, dadurch gekennzeichnet, dass sie den (die) weiteren Wirkstoff(e) in einer Menge von 0,01 bis 5,0 Gew.-%, vorzugsweise in einer Menge von 0,1 bis 0,5 Gew.-%, bezogen auf das Gesamtgewicht der kosmetischen Zusammensetzung, enthält.
12. Kosmetische Zusammensetzung nach einem der Ansprüche 1 bis 11, dadurch gekennzeichnet, dass sie außerdem Sorbitol, Harnstoff, ein Succinat, Ethanol, Dexpanthenol sowie übliche kosmetische Zusätze, Hilfsstoffe, Träger und dgl. enthält.
13. Kosmetische Zusammensetzung nach einem der Ansprüche 1 bis 12, dadurch gekennzeichnet, dass sie in Form einer Lotion, einer Creme, einer Salbe, einer Lösung, eines Gels oder eines Sprays vorliegt.
14. Verfahren zur Herstellung'der kosmetischen Zusammensetzungen nach den Ansprüchen 1 bis 14, dadurch gekennzeichnet, dass man einen oder mehrere Wirkstoffe der Formel (I) und/oder Tautomere und/oder Derivate davon einer in an sich bekannter Weise hergestellten Lösung oder Dispersion der übrigen Bestandteile der kosmetischen Zusammensetzung zusetzt, nachdem die Lösung bzw. Dispersion auf einen pH-Wert < 7,0, vorzugsweise zwischen 6 und 7, insbesondere zwischen 6,3 und 6,7, eingestellt worden ist, und das da- bei erhaltene Gemisch dann in üblicher Weise entlüftet, homogenisiert, kalt rührt und gegebenenfalls über einen Salbenstuhl laufen lässt.
15. Verwendung der kosmetischen Zusammensetzung nach den Ansprüchen 1 bis 14, insbesondere der darin genannten ADAPD-Wirkstoffe der allgemeinen Formeln I, la, lb, Ic, insbesondere von Methyl-ADAPD und/oder Ethyl-ADAPD, zur Verhinderung der Bildung bzw. zur Abschwächung oder Entfernung von braunen Hautflecken, insbesondere zur Behandlung von Melanosen, bei Mensch und Tier.
VI 2
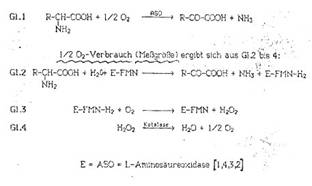
Y 20 inhibits
the amino acid-cleaving enzymes produced by enterobacteria (e.g. E. coli) such as amino acid oxidases
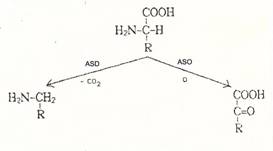
(ASO) and amino acid decarboxylases (ASD): Plate 11a.
Escherichia coli is hardly able to cover
its N requirement from non-protein sources (in contrast to lactobacillus).
Therefore, E. coli produces enzymes such as ASO, ASD et al.
which, in turn, "destroy proteins". Y 20 inhibits these amino-acid
cleaving enzymes and thus takes away sources supplying protein to E. coli.
Plate 11a: Scheme for amino acid-cleaving
enzymes such as amino acid oxidases L-ASO : (E 1.4.3.2)) and amino acid
decarboxylases (L-ASD)
This E. coli-inhibiting
activity of Y 20 is significant for its application in the animal feed
industry, because it involves regulation of the intestinal flora. Proof was provided
by the following experiments conducted in cooperation with Dr. M. Z. Abedin,
Higher Institute of Technology,
In vivo experiment with E. coli: The
ASO and ASD activities in the substrate of a liquid E. coli culture were measured both before and after the addition of
Y 20.
The result showed 61 % inhibition for ASO, and 25 % inhibition for ASD.
Tabelle
12b:
Füllplan
Manometer,
1 2 3 4 5 6
7 8 Füllort
Gefäß Nr.
L-Glu in Puffer -- -- 0,75
0,75 -- -- 0,75
0,75 HR
L-GDO in Puffer -- -- 0,25
0,25 0,25 0,25
0,25 0,25 SA
Y 20 -- -- -- -- 0,25
0,25 0,25 0,25
HR
Acetatpuffer pH 4,65 : 49,0
ml 1nNaOH + 100 ml 1n Essigsäure
L-Glu im Puffer :
1,0 mg L-Glu in 1,0 ml Acetatpuffer
L-GDO-Lösung :
125 mg L-Glutaminsäuredecarboxylase Typ III in 5 ml Acetatpuffer
Tabelle 12c: Meßwerte
1 2 3 4 5 6 7 8
Gefäßvol. 14,362
14,218 13,949 14,024
14,353 13,041
Zeit Min. 0
0
-1 +1 +1 0 0 0 0
10
+2
0 +72 +67 +111
+115 +101 +104
20
+4
+2 +87 +87 +
74 + 77
+ 54 +
60
30 +4
+1 +88 +92 +103
+105 + 74
+ 80
40
+3
+2 +88 +91 +108
+110 + 84
+ 90
h korr. +3
+84
+87 +216 +222
+181 + 190
µl CO2 109,9 274,9 252,9
(Mittelwert)
I: II: III:
getestet TB
L-GDO + L-Glu L-GDO + Y 20 L-GDO + L-Glu + Y 20
VI 3
Ascitomycetes taken into
consideration in the patents ref (5,7,8) and used as starting material for the
described preparations
[cf. following diagram 1 in IV 1]
Schizosaccharomycetes (millet beer in Afrika, also to be found in molasses),
endomyces lactis (milk mould, important for cheese maturing), saccharomyces (yeasts,
i.e. bakery-, brewery-, and
bottom-fermented yeasts), saccharomyces cerevisiae var.ellipsoideus (vine
yeasts), S. carlsbergensis (bottom fermented beer yeasts), S.fragilis JÖRGENSEN,
S.lactis DOMBROWSKI, S.rosei GUILIERMONT, S.biosoprus NAGARISHI,
Hansenula-yeasts as H.anomala HANSEN-SYDOW; trichosporon cutaneum OTA
(occurring in bakery and fodder yeast), Candida tropicalis and C.utilis
HENNEBERG L. and KR (Torulopsis utilis), C.Krusei (cream yeast) BERKHOUT;
Kloeckera JANKE (vine musts), Sporobolomycetes (grain, water, hay, straw),
Pichia polymorpha KLÖCKER (Pichia HANSEN).
VI 4
BSE detection: urine
test – ultrafiltration technique – BSE free countries
As soon as the first BSE-reports were published and the BSE crisis had
occurred, the author engaged in the following activities in
a ) In
co-operation with Dr.Pesserl the author looked for a method to detect BSE in
living animals as early as possible: Development of an ante-mortem diagnosis of
BSE-infected cattle (1986-89)
b ) The
author checked ultrafiltration technique to eliminate “infectious
BSE-components” – in co-operation with the dres.Faria and Pesserl
c ) He identified countries which were free of BSE such as
re a) The comparison of the “protein profiles of the urine” of
healthy cattle with the urine of cattle infected with BSE made BSE detection
possible (Riemschneider, Pesserl 1989) and has been put to use succesfully by
us at that time: Plate 12.
After the author had learned that a “Research Institute
for Animal Epidemics” had been established on the
re b) In a 34 page expert opinion of CONSULTING
DEVELOPMENT ENGINEERING in 1993 regarding the absence of BSE in the proteinfree
thymus extract OMNITHYMUS, Charge STK 1993-1, initiated and supervised
by the author, the activity of the ultra
filtration technique employed for this extract was established, cf. 3 documents: i) Summary, ii) list of contents of the 1993 expert
opinion, iii) certificate regarding the examination of the OMNITHYMUS extract
supplied to Japan in 1996 concerning infectiousness and a possible content of
BSE prions: BSE-free (next pages).
Until December of the year 2000, i.e. until the import ban imposed by the Japanese
government came into effect, preparations produced from calf thymus serum in
- a urine test
- proving
that the ultra filtration technique
used during the manufacture of the preparations was effective; see documents
belonging to i) and ii) next page
- and,
where possible, obtaining the animal organs
from BSE-free countries.
Since no prions were found in porcine organs between
1986 and 2008, requests for proteinfree porcine organ extracts and porcine
collagen increased from 2001 onwards and could be fulfilled (3).
As early as the 1950s, the author
had developed extracts from porcine placentae for the Brazilian industry (3),
i.e. long before bovine placenta extracts became en vogue; see the publication: "Porcine-based organ extracts
guarantee full substitution of bovine extracts" (23).
Plate 12: Announcement of
the round-table conference in Rio de Janeiro, directed by M.M.Faria,
Chairman of CONSULTING DEVELOPMENT ENGINEERING:
Invitation to a round-table
conference on the subject:
“Ante-mortem diagnosis
of BSE-infected cattle”
In the library of CONSULTING
DEVELOPMENT ENGINEERING, in
Initiator and project leader:
Professor Dr.Dr.R.Riemschneider, member of the Brasilian University
Universidade Federal de Santa Maria (UFSM), in Santa Maria, RS, Brazil, and
retired professor of the Free University of Berlin (FU), Institute of
Biochemistry. Dr.F.R.Pesserl is directing the BRASTONE Company in Curitiba, PR,
Brazil, acting as lecturer giving the report of the mentioned authors
Riemschneider and Pesserl, in Portuguese, entitled as above written.
It will be presented the new ante-mortem
test based on the body fluid urine applying the method of special
gel-electrophoresis * to compare the
urine protein profiles of cattle – including experimental data in detail. The
authors used the urine of 10 calves, 5 of them infected with BSE at 3 months of
age, 5 of them as controls; during 18 months urine samples were taken nine
times.
Rio de Janeiro, November 1989
This round-table talk is not
public – only for invited guests to be kept secret
______
*
after suitable preparatory
treatment of the urine samples (instantly deep frozen after taking)
We refrained then from any publication for following reasons:
- a) to
avoid complications with the Brasilian Health Department because of the
experiments with BSE infected material in
- b) to avoid also complications
with the rather inflexible German authorities.
- c) the
author remembered well the attacks of the radical students, starting 20 years
ago (68), and will never forget the “science-destroying” new university law of
1969 concerning the so-called democratisation of the FU and the consequences –
so that the author found himself compelled to transfer some of his current
important research projects to Brazil and to call on highly industrial help in
Brazil and other countries, especially Japan.
Three documents (mentioned in VI 4)
i) Summary of the 1993 expert
opinion
regarding the ultrafiltrated thymus
extract OMNITHYMUS[4] tested on infectious
properties and possible BSE-prions – test animal: Syrian golden hamster,
observed 365 days, microscopical anatomic inspection of their brain – 34p
report.
This report and the report iii) were initiated and
supervised by the author who worked yearly some months in
It is quite important to point out that in
Result: Extract, free of infectious particles like
scrapie.

ii) List of content of the OMNITHYMUS
expertis [summary in i)]

Long-winded and complicated expertises like the above documented one and
preliminary BSE-tests made it possible to continue with the production and
export of animal based preparations used in medicine and cosmetics - without
any interruption – in spite of the BSE-crisis.
Reading the 34 pages document with one year lasting Syrian golden hamster experiments you must take
into consideration the situation of 1985/86: In England, thousands and
thousands of cows had to be clubbed, in spite of the prohibition calves were
exported to Holland and other countries; the nature of the pathogen particle
responsible for BSE was not known then; there was a certain similarity of the
symptoms of BSE with the JAKOB-CREUTZFELD syndrome and scrapie (Australia). Summarized under the term TSE = transmissible
spongiforme encephalopathies
All this then called for immediate action – cost what
it may.
iii)
First
page of the 1996-Certificate -
regarding the examination of OMNITHYMUS, prepared according to the
prescription of the author - 33 pages report, similar to i) and ii).
Result: Extract free of infectious particles like scrapie
or scrapie-likes.

Conclusion
The methods of preparation described have been used successfully in
different fields, especially in combination with each other, but also in
combination with native preparations. As mentioned above, a pharmaceutical
preparation on the basis of a synthetic organ extract has been put to clinical
use as long as 14 years before the
BSE crisis.
Based on the patents described, the following preparations were
developed:
Cytocatalyzers as proteinfree cosmetic additives: yeast-based
Seruryel, Tricell
as proteinfree cosmetic additives: cereal-based
Colezzo as food-drug: cereal-based
H 2000, Y 2000
as animal food additive (yeast-based)
Aphrodisiac
(yeast based)
Collaplant PO
as collagen substitute (glycoproteine-based)
Canivit for dogs (yeast based)
Omnithymus
S, a synthetic
proteinfree extract (thymus analogon)
Depending on requirements, each of the three patent groups (5,7,8) achieved
optimal solutions. The author has investigated the “active principles” of the
reaction products received by the methods described in these patents (5,7,8).
The results will be published later. In the EU-patent (1) these “active
principles” are already used; see the component description a) - c) in (1).
The patent (16) permits an increase of the whitening effect of synthetic
or native preparations.
REFERENCES - by the
author (co-workers in brackets)
(20) Re-reading - 66 years chemistry: XXVI PROJECTS (in
preparation) here: PROJ XXII and XXIII
(21a) The negative influence of regular intact
yeast (baker's yeast) on the potency of male mice when given with the drinking
water.
Lab report June 1938, 15 pages,
abbreviated version published in 1938 in the school magazine of Wilhelm
Gymnasium, Hamburg-Dammtor (co-workers: A. Suhr, H. Kahl)
These orientation experiments were
carried out during the period May 1937 to June 1938 with the approval of the
administrative board of Matthias-Claudius-Gymnasium, financed by the family of
the author's schoolmate A. Suhr from Wilhelms-Gymnasium.
(21b) "Yeast and yeast extracts -
Saccharomyces cerevisiae HANSEN – BIOCOMPLEX YEAST"
Lecture held by the author in December
1939 at the Chemical Colloquium of the Department of "Analytical
Chemistry" of the Chemical Institute of Göttingen University (discussion
chaired by Profs. Dr. J. Goubeau and Dr. G. Rienäcker), duration of the
lecture: 60 minutes.
General lecture, state of the literature until 1938 by
Chemical Abstracts, jointly prepared with A. Suhr (student of medicine) and A.
Kersting (student of chemistry)
On the topic: BIOCOMPLEX YEAST: No natural product
deserves being called a bio-complex more than yeast. State-of-the-art chemical
analyses have shown that beer yeast or baker's yeast, respectively, are the plants with most active ingredients we know
so far; possibly to be compared only with cereal grain [e.g. rye, wheat (11,28,29)];
cf. PROJECT XXII in (20).
(22) Lecture
"Test method for examining aphrodisiacs: First tests for identifying and
isolating the "factor of yeast inhibiting the potency of mice", held by
the author at the Colloquium of the Institute for Physiological Chemistry at
Berlin University in April 1948 [discussion chaired by Prof. Dr. K. Lohmann,
discoverer of ATP; cf re 5) KARL LOHMANN in (15)].
The results of "Aphrodisiacs", Comm. I to VIII
(20), were presented and discussed. By special invitation of Prof. Lohmann, the
following were present:
- Nobel
Prize winner Otto
- Prof. Dr. J. Just,
- Dr. A. Heymons, Director of Riedel de Haen,
Berlin,
- Prof. Dr. E. Thilo, Director of the Institute
for Inorganic Chemistry of
- Prof. Dr. K. Noack, Dean of the Department of
Mathematics and Natural Sciences at Berlin University and Director of the
Botanical Institute at the same address.
In this address to the above-mentioned personages, the
author first spoke in public about the "unexpected results" of the
WARBURG experiments obtained with such "yeast extracts" he had
prepared while "looking for the factor inhibiting the potency of
mice"; a very high
respiration-increasing factor of 3 to 4 had been found for these plant extracts. Under corresponding test
conditions, the factors found for animal extracts
(like extracts from foetal calf serum) were only between 1.8 to 2.4 (12-14);
cf. PROJ. XXIII (20).
More details on the communication series "Aphrodisiacs"
are found there, too.
(23) Porcine
based organ extracts guarantee full substitution of bovine extracts
http://www.vevy.com/relata/issues.articles 2001, 3p
[co-author: M. Heisler]
International Electronic Journal on
Dermopharmacological Research, Dermopharmaceutical Technology and Related Cosmetic Subjects
(24) “The animal food additive: S
2000 (Y 2000), based on Y 20-suspension” spray dried”
Lecture given in
- invited competent Russian scientists from
the different parts of the
- industrialists (fodder industry),
-
fur farmers,
-
authorized persons of the Government.
-
bankers and German guests
The lecture was held by
the author two times in the aula of the Deutsche Bank and simultaneously
translated into the Russian language, 120 min, 25 diapositives. Summary of the
lecture in Plate 5.
The author handed over
the special report mentioned in (8c)
to everybody.
(25) Lecture
identical with (24), held in
Present: Professor Dr. Czeslaw Lewicki,
Agricultural and
from
BACUTIL: Direktor J. Krzaczek, Gdansk
Negotiations for Y 2000, continued succesfully in Berlin on Sept 19 and 20, 1989 with the
Polish delegation of Landwirtschaftl.-Technische Akademie,
Olsztyn: Prof. Cz. Lewicki, Prof. A.
Faruga, Mgr. D. Mikulski, Zootechn. Institut, Krakau: Dr. Doc. J. Koralewski
Akademie der Wissenschaften: Jablona/Warschau Dr. B. Pastuszewka Zentrallabor
für Futtermittel, Lublin: Dr. St. Blaziak;
BACUTIL,
Gdansk: Dir. J. Krzaczek, NAVIMOR: Dir.
K. Krzymowski, Tomaszewski and Mgr. L. Drobotowicz, lawyer
Prof.Riemschneider repeated his lecture held in
Mikulski, Koralewski, and Krzaczek reported about “Massentierzuchtversuche
mit Y 2000 and a running chicken field
experiment with 6000 t added the new food animal additive Y 2000;
Mrs Pastuszewaka spoke about her lab trials with
mkice, rats, rabbits, fur animals;
Mr Blaziak about results with pigs and fish.
(26) Lecture identical with (24), held in
Discussion of the pig experiments carried out in
Daraufhin
sind in der Zeit vom 20.4. – 14.6.1990, initiiert durch diesen Vortrag, mit H
2000-Prämix
(20
% Y 20) – genannt PROVAL - in der Produktions-gemeinschaft Gyözolom in
Lajoskomárom (Schweinemästerei) mit 306 Nagy magyar fehér + Sved lapály [große
ungarische Weisse u. Schwedische Niederung] Ferkel-Versuche durchgeführt
worden. Die brasilianischen Versuchs-ergebnisse aus den Jahren 1969 bis 1970
konnten bestätigt werden.
(27) Three lectures in
invited from the Comprehensive Agricultural
Corporation, Tianjnin; simultaneous tranlated into Chinese language
Lecture
I and II: “S 2000 als Futtermittelzusatzstoff zum
Produkt Feed BE Yeast,
einer
chinesischen Futterhefe – Eigenschaften und Ergebnnisse der Feldversuche mit
Hühnern, Ferkeln und Rindern aus Brasilien, Japan, USSR (24), Polen (25), Ungarn (26) und der
Schweiz.“ Present:
- Mr.
Li Feng Zhang, Chairman of the board – General Manager Hua Da Forage Industrial
Corp., Tianjin
- Mr. Zhou Zhijiang, Professor and Vice-President of the Preparatory
Committee of Man-made Fiber Industry
Association China
- Mr.
Liu Baoshun, Associate Professor, Beijing Research Institute for Nutritional
Resources
- Mr. Wang King Min,
Attead Office Director, Daqui Comprehensive Agricultural Corporation Tianjin
- Mr. Yu Sao Wu, Deputy
Manager, Hua Da Forage Industrial Corporation, Tianjin
- Mr. Song Bao, Deputy
Manager Factory Director, Daqui Poultry and Aquatic Breeding Farm, Tianjin
- Mr.
Kauer, Kauer-Industrie Consult and Trading GmbH, Berlin
- Mrs. Kauer-Ong of
Kauer Industrie Consult and Trading, also interpreting (German, English, Chinese)
Lecture III : „Probiosis versus Antibiosis“
After lecture II a long discussion took place about
the results of the field experiments
concerning the combination of BE with S 2000 (FEED BE – an animal feed yeast produced in
The last mentioned lecture was given for the first
time on Sept 1963 in
(28) Preparation
of different plant extracts and test on their cell metabolism increasig
properties. Extracts from: rye, wheat, cactus, tea, papaya, ginseng ecc
Lab reports, from Dez 1947 on
(29) Intermediate
metabolism – organ and plant extracts
Bull. I –
here:
Bull.X:
Cell culture experiments: Wound healing by plant extracts like wheat, rye,
ginseng, cattail, 15 series experiments,
ms
1973 (with R.Lipp, W.H.Chik)
Bull. XLV: Plant extracts from placenta of
triticale, rye, wheat, corn, barley, oat, rice ecc - preparation and properties
- interesting for cosmetics: CELLRYEL (SERURYEL)
ms
Dec 1987, 22 p (secreted)
Bull.
XLVI : New cosmetic additives based on rye and wheat: CELLRYEL, WHEATIN –
activity data (with U.Rotter, M.Azhar)
- first draft of a patent application.
Ms Jan 1988
Expert opinions about activity of
CELLRYEL by Hwa Sook Chang, Hongkong, by M.Azhar,
In memoriam:
This article is dedicated to the author’s
long-standing Brazilian friends M.M. Faria and J.M. Faria, who unfortunately
died in the crash of a private plane belonging to colleagues.
[1] Adress
correspondence to Prof. Dr. Dr. R. Riemschschneider, D-14001 Berlin, Postfach
1164, Germany
[2] BSE = bovine spongiforme
encephalopathie (10)
[3] See Plate 8b (claim 27)
[4] Die vom Verfasser für
OMNITHYMUS entwickelte Herstellungsvorschrift umfasste folgende Stufen:
Zerkleinern der gewaschenen Drüsen - Suspension in Ethylether bei -20° C -
Zentrifugieren (explosionssichere Trommelzentrifuge) – Dialyse des
Zentrifugats – Standardisierung – Sterilfiltration. Ab 1986 ist dieser, seit
1970 nach Japan exportierte proteinfreie Organextrakt zusätzlich ultrafilriert
worden (Gutachten); später wurden darüber hinaus noch Thymusdrüsen verwendet,
die aus einem BSE-freien Land stammten (bis zum Inkrafttreten des neuen
Gesetzes im Dezember 2000).
[ BWW Society Home Page ]
© 2009 The Bibliotheque: World Wide Society